Learning and Memory in an Animal Model of Longevity: The Ames Dwarf Mice
Abstract
The Ames dwarf mice have a recessive mutation of the PROP-1 gene that produces hereditary dwarfism. The abnormality is responsible for an anterior-pituitary deficiency that results in a substantial reduction of growth hormone, thyroid-stimulating hormone, and prolactin. These mice are smaller in size than their normal siblings but live approximately twice as long. The normal siblings do not have the mutation, and therefore still have the typical levels of the three hormones. The purpose of the present research was to determine if the reduced hormones in the Ames dwarf mice affected their ability to learn and delayed the age-related loss of memory. In general, the hypotheses proposed indicate that there will be no significant differences on the tasks in regards to the genotype or the age of the mice. These hypotheses would support previous research and suggest a delay in the age-related loss of memory and the ability to learn in the Ames dwarf mice. Learning was assessed using a matching-to-sample procedure, while memory was evaluated using a modified radial-arm procedure. Generally, the age of the animals had little to do with their performance on any of the tasks. Taken together, the overall results showed no significant differences in accuracy between any of the groups of mice or a behavioral decline as the mice age. The present results are consistent with the theory of a delayed age-related behavioral decline in the Ames dwarf mice.
Author Contributions
Academic Editor: Anubha Bajaj, Consultant Histopathologist, A.B. Diagnostics, Delhi, India
Checked for plagiarism: Yes
Review by: Single-blind
Copyright © 2025 David P. Austin, et al.
 This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Competing interests
The authors have declared that no competing interests exist.
Citation:
Introduction
In the early 1960s, researchers discovered a recessive mutation that caused hereditary dwarfism in mice 1. The mice are smaller in body size but live significantly longer (49 & 64%; males & females, respectively) than their normal siblings 2, 4. Ames dwarf mice have a point mutation of the PROP-1 gene expressed on chromosome 11 5. The mutation prevents appropriate differentiation of cell types in the anterior pituitary resulting in deficiencies in plasma growth hormone, thyroid-stimulating hormone, and prolactin 3. The lack of specific circulating hormones has been implicated by many researchers as partially responsible for the enhanced longevity, as the endocrine system is believed to participate in the aging of a number of organ systems 3, 6. Thus, prior research suggests that extending the normal life span in mice could be possible by reducing specific hormones and may be applicable to humans 3. However, before scientists begin conducting controlled experimentation by reducing human pituitary hormones, more research needs to be conducted on both the potential risks and benefits of such a reduction. Ames dwarf mice provide a good avenue of examination, specifically on the operant effects of a hypo-pituitary-induced longer life. Age-related behavioral decline has been well documented in normal mice. However, little behavioral data on the Ames dwarf mice has been reported 7, 8.
Previous Work
A literature search on the Ames dwarf mice yielded numerous biological and physiological based manuscripts, but led to only two studies on the behavioral effects of a hypo-pituitary induced longer life. The first, Kinney et al. (2001) examined the Ames dwarf mice against normal controls on a variety of behavioral tasks. The study used a total of four groups of animals; two young groups and two old groups. The two young groups consisted of Ames dwarf mice and normal controls, the ages of which were in the 3-5 month range. The old Ames dwarf mice were 36 months old, while the old normal mice were 19-21 months old. The ages of the animals in the old groups were chronologically different, but physically similar. Biologically, the mice in the old groups had all lived for approximately the same percentage of their expected life-span 7.
The behavioral tasks used by Kinney et al. (2001) included using an elevated plus maze to measure anxiety, an inhibitory avoidance task to measure long-term memory, and locomotor activity meters to measure spontaneous movement. The results of the study revealed that the old Ames dwarf mice and the old normal mice both had a comparable decline in anxiety levels examined against the younger groups, as measured by their behavior in the elevated plus maze. On the inhibitory avoidance task, the old Ames dwarf mice did not differ from the young groups in their performance. However, the old normal mice performed worse than both of the young groups. Finally, the locomotor activity meters revealed that the old Ames dwarf mice did not have any age-related spontaneous movement decline compared to their younger counterparts. Taken together, these results suggest a delay in the age-related deficits in the Ames dwarf mice 7.
The second behavioral study using the Ames dwarf mice employed an open-field test to indirectly measure locomotor activity 8. The study utilized four groups of Ames dwarf mice, with approximately ten animals per group. The four groups included the young males and young females, both of which were 2-4 months old, and the old males and old females, which were 12-16 months old. The mice were placed in an open-field Plexiglas box that had marked squares covering the floor. The number of marked squares that the mouse entered was recorded, as was the total time spent in each square. Each trial lasted five minutes. The results revealed that the old Ames dwarf mice entered more squares and spent more time in each square, suggesting that the old mice were more active than the young mice. There were no sex differences found for any of the measures 8.
The purpose of the present study was to further the understanding of the aging process. The current research uses different behavioral procedures to evaluate an animal model of longevity and to identify a possible hormonal foundation for changes in behavior. The hypotheses of the present investigation were developed to support previous behavioral research involving the Ames dwarf mice, suggesting a delay in the age-related loss of memory and the ability to learn. Therefore, this study hypothesizes no significant differences on any of the tasks with regards to the age of the mice. In addition, no significant differences are thought to be discovered between the different age groups of the mice.
Method
Power analyses were conducted for the present research using the GPOWER program 9 following the suggested procedures outlined by 10. Calculations were based on a desired power of 0.80 using an effect size of 0.25. The effect size was used to detect a medium effect as reported by 11. Phase I of the study utilized 37 mice (six to eight per cell) which each ran 10 sessions. A total sample size of 34 mice (six to eight per cell), which each ran 20 sessions, were used in Phase II. Based upon the nature of each design, the desired power was adequately produced at Phase I (N=37) with a result of 0.98 and at Phase II (N=34) with a result of 0.97.
Animals
The current study investigated learning and memory in Ames dwarf mice compared to their normal siblings. The Ames dwarf mice are created by mating homozygous (df/df) or heterozygous (df/+) dwarf males with carrier females (df/+), which produces an anterior pituitary deficiency. The normal siblings are produced without the anterior pituitary deficiency 9, 7. The study utilized 37 mice (eight young female Ames dwarf mice (3-5 months), eight young male normal siblings (3-5 months), six old female Ames dwarf mice (18-21 months), seven old female normal siblings (18-21 months), and eight very old female Ames dwarf mice (32-35 months). Three of the mice died during data collection (one young normal mouse between Phase I and II and two very old Ames dwarf mice during Phase II). Most of the mice used for this research were female. However, due to the availability of obtaining the mice, one group (young normal) of mice were male.
All of the mice were housed in groups according to their age and genotype. The mice were housed in the same room where the research occurred. The lighting conditions of the room were kept on a 12:12 h light/dark cycle (7am to 7pm) and the temperature remained relatively constant at 22° ± 2º C. The mice had unrestricted access to water via a 12-oz bottle with an extended sipper tube in each enclosure. The mice were given a daily feeding of an 18% protein rodent maintenance diet (Teklad Global) at least 30 min after their session that lasted for approximately 2h. During the days when data were not collected (weekends, holidays, etc.), the mice were put on a free-feeding procedure. The feeding protocol was necessary to maintain weights at approximately 85% of their free-feeding control weights (or normal weight minus 15%). Before the mice arrived at the laboratory, they were on a free-feeding protocol. Therefore, the control weights were determined by weighing the mice directly upon arrival to the lab.
Apparatus
Phase I
The experimental apparatus for Phase I was a mouse hexagonal hub (Coulbourn Instruments; Model H10-35R-08) with a grid floor. The hub was placed in an isolation cubicle (H10-24T) for protection against background noise on a table approximately 70 cm above the floor. On three consecutive walls of the hexagonal hub, there was an aperture (H14-01M) measuring 2.2 cm (H) X 2.2 cm (L) which was even with the grid floor that was used as a nose-poke operandum. A photocell sensor (H20-93A) at this location was equipped to detect entrance into the mechanism. Positioned above the center aperture, were two three-light panels (H11-02M) with LED lights that measured approximately 2.5 cm (H) X 5.0 cm (L) which were used for stimuli. One three-light panel was located above each peripheral aperture. To ensure maximum discrimination, the top three-light panel was associated with the left peripheral light panel, while the bottom three-light panel was associated with the right peripheral light panel. Based on the conditions, the lights could be constantly illuminated or pulsated at the rate of 1s on, 1s off. The reinforcer of 0.02 cc of a 0.02% saccharin solution was delivered by a solenoid liquid dipper (H14-05M), one in each of the two peripheral apertures. In addition, the Graphic State (Coulbourn Instruments) computer program created data files for each mouse in order to record all events.
Phase II
The apparatus for Phase II of the study was a hexagonal hub (Coulbourn Instruments; Model H10-35R-08) that was similar to that used in Phase I. The hexagonal hub was modified into a variant of the radial-arm maze, a deviation of the maze developed by Olton and Samuelson (1976). The new variant used four solenoid liquid dippers (H14-05M) located on four opposite ends of the hub. The apertures (H14-01M) to each liquid dipper measured 2.2 cm (H) X 2.2 cm (L) with the openings positioned even with the grid floor. The liquid dippers delivered a reinforcer of 0.02 cc of a 0.02% saccharin solution when the proper conditions were met. In addition, photocell sensors (H20-93A) were equipped to detect the mouse entrance into the aperture. The Graphic State (Coulbourn Instruments) computer program created data files for each mouse in order to record all events.
Procedure
At the beginning of each experimental session, a mouse was placed in the hexagon hub. The hub was closed and the isolation chamber secured. The researcher then initiated the experimental protocol on the computer. After the session was completed, the researcher removed the mouse, weighed it, and placed it back into its housing unit. Each mouse was run at approximately the same time of day, five days a week.
Phase I
The Phase I trials were designed to measure the ability of the mice to acquire an operant response (nose poke) using a simultaneous matching-to-sample procedure. All 37 of the mice were evaluated in the initial phase. At the beginning of the simultaneous matching-to-sample procedure, one of the center lights was illuminated (constant or flashing) by itself until the mouse performed an observing response (i.e., a nose poke in the center aperture). If the mouse failed to perform the observing response within 10s, the trial ended and a 10s inter-trial interval began. Once the mouse performed the response, the comparison stimuli side lights were illuminated, one of which was an indistinguishable stimulus and the correct match. The mouse was then required to nose poke the correct corresponding aperture, after which the liquid dipper would raise for a reinforcement period of 6s. If the incorrect aperture was entered, the comparison stimulus associated with the incorrect nose poke was terminated. The correct response initiated a 10s inter-trial interval that separated successive trials. The presentation of the lights (constant or flashing) was random, but programmed so that each would occur 50% of the time. Sessions ended after the mouse received 20 reinforcers or 5 min elapsed without a response. A total of 10 sessions were used in order to evaluate Phase I.
Phase II
Phase II of the study investigated memory in Ames dwarf mice compared to the control mice using a modified radial-arm procedure. Potential differences were evaluated using a total of 34 mice, which included eight young female Ames dwarf mice (3-5 months), seven young male normal siblings (3-5 months), six old female Ames dwarf mice (18-21 months), seven old female normal siblings (18-21 months), and six very old female Ames dwarf mice (32-35 months). As noted above, one of the young normal mice died between Phases I and II. In addition, two of the very old Ames dwarf mice died during data collection in Phase II and their data were not included in the analysis.
The results of Phase II were measured using a modified radial-arm maze procedure in which .02 cc of .02% saccharin solution reinforcement was available in each aperture located on four opposite walls of the mouse hub. On each trial, the first visit to aperture 1, 2, 3, or 4 was reinforced, but successive visits were not. The next trial began once every aperture had been visited, and was signaled by 6s of flashing by the house light. Sessions ended after the mouse completed 10 trials or 10 min elapsed without a response. A total of 20 sessions were conducted in Phase II. The animals were performing consistently up to 20 sessions, therefore data collection was ended.
Dependent Variables
Phase I
The dependent variables used to assess Phase I included the session duration, trials completed, errors, and accuracy per session. Session duration was defined as the amount of time, in minutes, that each mouse used to complete one full session. Session duration was used to understand if the animal performed the procedure efficiently or if the animal was mostly inactive during the session. The trials completed were the number of trials that each mouse finished during one full session. An error was defined as an incorrect response following the initial orienting response on each trial. Finally, accuracy was defined as the percentage of correct responses over one full session in Phase I.
For all of the DVs in Phase I, there were 10 experimental sessions that were grouped into two 5-session blocks to test for learning across sessions. Several prior studies have also used 5-session blocks to test across sessions 12, 13, 14.
Phase II
The dependent variables used to assess performance in Phase II included the session duration, trials completed, and errors per trial. Session duration was defined as the amount of time, in min, that each mouse needed to complete one session. Again, the session duration shows whether the animal performed the procedure efficiently or if the animal was inactive during the session. The trials completed are the number of trials that each mouse needed to complete a session. Trials completed, combined with the session duration, show if the animal performed the procedure efficiently. Errors were defined as unrewarded repeat visits to an aperture before the next trial began. Errors per trial were the number of repeat visits per trial averaged over the session. Errors per trial were assessed to evaluate if the animal learned the procedure. For all of the DVs, there were 20 experimental sessions that were grouped into four 5-session blocks to assess learning over time.
Results
Inititally, a series of repeated-measures ANOVAs were performed to identify whether the Old Ames dwarf mice and the Very Old Ames dwarf mice differed in performance on any of the behavioral measures. The analyses revealed that there were no statistically significant differences between the two groups on any of the tasks performed during Phase I or II (all Fs<1.00, all ps>0.05). Therefore, for both measures, the Old Ames dwarf mice and the Very Old Ames dwarf mice were combined into one group for simpler analysis (referred to as the Old Ames dwarf mice).
The data was further analyzed using a three-way (Genotype X Age X 5-Session Block) mixed-model MANOVA. Genotype and age of the mice served as the between-subjects variables, while the five-session block served as the within-subjects variable.
Phase I
Following the analysis of the matching-to-sample procedure, a statistically significant main effect of genotype was observed, Pillai’s Trace F(3,32)=3.70, p=0.01, Cohen’s f=0.18. Subsequent univariate analyses revealed that the effect of genotype was significant for session duration, F(1,32)=5.21, p=0.02, Cohen’s f=0.10, with the Ames dwarf mice running shorter sessions (see Figure 1). The Ames dwarf mice completed fewer trials, F(1,32)=13.23, p=0.00, Cohen’s f=0.36 (see Figure 2) and made fewer errors than the controls, F(1,32)=14.34, p=0.00, Cohen’s f=0.37 (see Figure 3). The mice performed similarly in terms of accuracy, F(1,32)=1.45, p=0.24, Cohen’s f=0.00.
Figure 1.Session Duration (Phase I)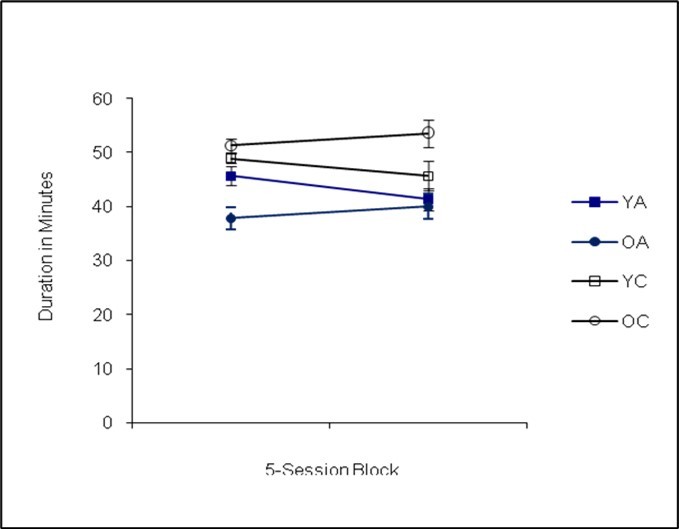
Figure 2.Trials Completed (Phase I)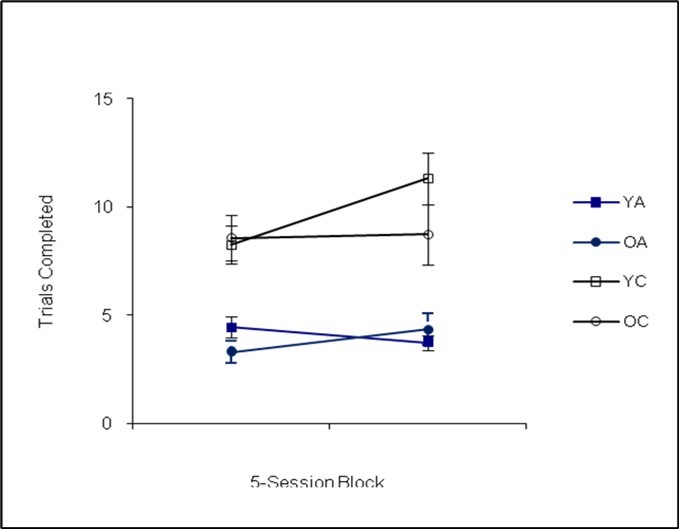
Figure 3.Errors per Trial (Phase I)
The results did not indicate a main effect of age, F(3,32)=0.53, p=0.72, Cohen’s f=0.13 or a main effect of 5-session block, F(3,32)=1.71, p=0.16, Cohen’s f=0.11. Furthermore, the results did not show an overall interaction between genotype and age, F(3,32)=0.84, p=0.51, Cohen’s f=0.09.
Phase II
An analysis of the modified radial-arm maze procedure revealed a statistically significant main effect of genotype, Pillai’s Trace F(3,30)=21.14, p=0.00, Cohen’s f=0.35. Additional univariate analyses revealed the effect of genotype was significant for session duration, F(1,30)=26.38, p=0.00, Cohen’s f=0.14, with the Ames dwarf mice running longer sessions (see Figure 4). The Ames dwarf mice completed fewer trials, F(1,30)=14.91, p=0.01, Cohen’s f=0.17 (see Figure 5), and made fewer errors per trial than the controls, F(1,30)=7.58, p=0.01, Cohen’s f=0.17 (see Figure 6).
Figure 4.Session Duration (Phase II)
Figure 5.Trials Completed (Phase II)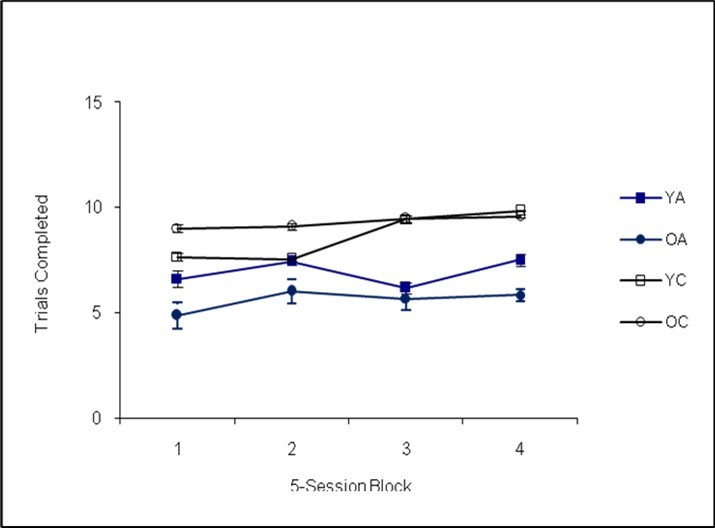
Figure 6.Errors per Trial (Phase II)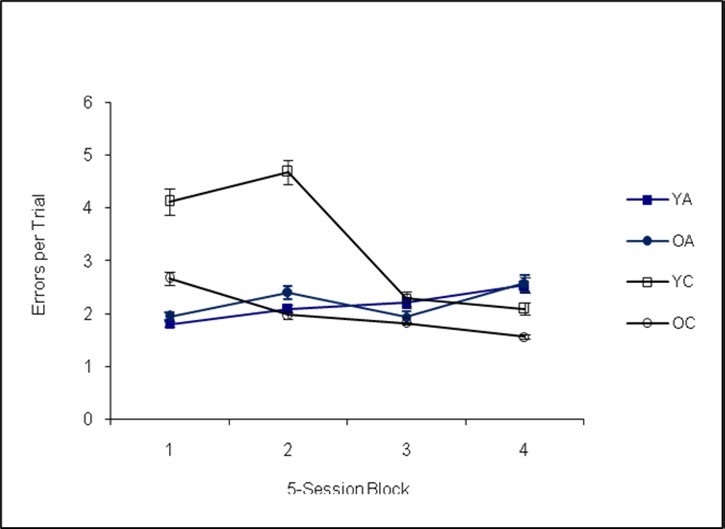
The statistical results did not indicate a main effect of age, F(3,30)=1.84, p=0.16, Cohen’s f=0.14. The main effect of 5-session block was also not statistically significant, F(3,30)=1.59, p=0.12, Cohen’s f=0.10. The results showed an overall interaction between genotype by age, F(3,30)=5.10, p=0.01, Cohen’s f=0.18, but subsequent univariate analyses showed that none of the differences reached statistical significance for the individual dependent variables (all Fs<3.20, all ps>0.05).
Discussion
For most of the behavioral measures assessed in the present study, age appeared to have little impact on performance. After examining the combined results across all measures, no significant differences emerged in accuracy between any of the mouse groups, nor was there evidence of behavioral decline with age. These results may align with the theory of a delayed age-related behavioral decline in the Ames dwarf mice, however, the interpretation is limited due to overall accuracy rates near 50%.
Another main finding of the current study showed an effect of genotype. However, closer analysis revealed the Ames dwarf mice performed just as accurately as the control mice across all of the behavioral measures. The primary difference was that the Ames dwarf mice did not complete as many trials per session as the control mice, indicating slower task performance. This result suggests a possible size limitation with the Ames dwarf mice. Again, the young Ames dwarf mice were approximately one-third the size of their normal siblings. Using regular mouse equipment, the Ames dwarf mice would have a more difficult time performing an operant response. The dwarf mice would have to crawl up into the nose poke to perform a response and they would need to crawl into the dipper aperture to receive reinforcement. To address the size concern, the operant chambers were modified by lowering the apertures to floor level, improving access for the dwarf mice.
Another question concerning the size of the mice was that the smaller the mouse, the quicker and easier it was to become satiated. The increased susceptibility to satiation might help explain the lower rate of responding in the Ames dwarf mice. To address this issue, the researchers used a liquid reinforcer of .02 cc of .02% saccharin solution instead of a food-pellet reinforcer. The researchers expected that mice were unlikely to become satiated by the relatively small number of liquid reinforcers they could receive in any given session. Still, the smaller size of the dwarf mice is an important consideration when designing further studies involving these animals.
Conclusion
Understanding the aging process requires thoughtfulness and consideration of various factors involved. Using mouse models of aging, scientists can better understand the process and control for several unknowns. The Ames dwarf mice provide an understudied avenue of research that involves the hormonal impact of aging. Using the Ames dwarf mice in behavioral studies will contribute to the scientific community by improving our knowledge of the hormonal involvement of the aging process. More behavioral studies utilizing the Ames dwarf mice need to be conducted to help clear up any inconsistencies in results and to further scientific study in the area.
The Ames dwarf mice, with their substantial lack of growth hormone, thyroid-stimulating hormone, and prolactin, performed equally in accuracy on the measured tasks as the control mice. In fact, the results show no statistically significant age-related differences on any of the tasks. However, some of the age-effects of the mice on the matching-to-sample and maze procedure were marginally significant. Although the Ames dwarf mice did perform similarly to the control mice, their performance on each of the procedures was extremely poor. Those present findings results are therefore inconclusive regarding the hormonal impact of aging.
Future research should aim to investigate, in greater depth, the behavioral impact of the reduction of anterior-pituitary hormones using the Ames dwarf mice. It is important to carefully consider many of the limitations and concerns of the present study and the Kinney et al. (2001) study to create additional well-rounded contributions in the area of the hormonal impact of the aging process.
References
- 2.Bartke A, Brown-Borg H. (2004) Life extension in the dwarf mouse.Current Topics in Developmental Biology. 63-189.
- 3.Bartke A, Brown-Borg H, Mattison J, Kinney B, Hauch S et al. (2001) . Prolonged longevity of hypopituitary dwarf mice.Experimental Gerontology 36-21.
- 4.H M Brown-Borg, K E Borg, C J Meliska, Bartke A. (1996) Dwarf mice and the ageing process.Nature. 384-6604.
- 5.M W Sornson, Wu W, J S Dasen, S E Flynn, D J Norman et al. (1996) Pituitary lineage determination by the prophet of pit–1 homeodomain factor defective in Ames dwarfism.Nature. 384-327.
- 6.Brown-Borg H M Rakoczy, G S. (2003) Growth hormone administration to long-living dwarf mice alters multiple components of the antioxidative defense system. , Mechanism of Ageing and Development 124, 1013-1024.
- 7.B A Kinney, C J Meliska, R W Steger, Bartke A. (2001) Evidence that ames dwarf mice age differently from their normal siblings in behavioral and learning and memory parameters.Hormones and Behavior. 39-277.
- 8.B A Kinney-Forshee, N E Kinney, R W Steger, Bartke A. (2004) Could a deficiency in growth hormone signaling be beneficial to the aging brain?Physiology and Behavior. 80-589.
- 9.Erfelder E, F Buchner, A. (1996) GPower: A general power analysis program. , Behavior Research Methods, Instruments, & Computers,28 1-11.
- 10.J L Myers, D A Well. (2002) . Research design & statistical methods, 2nd ed. Mahweh, NJ: Lawrence Erlbaum Associates .
- 12.S W Aum, B L, N S Hemmes. (2003) The effects of concurrent task and gap events on peak time in the peak procedure.BehaviouralProcesses. 65-1.
- 13.J, M P Gillam, M G Paule. (2003) The effects of prenatal cocaine exposure on reversal learning using a simple visual discrimination task in rhesus monkeys.Neurotoxicology and Teratology. 25-4.
