Clinical Evaluation of Significance of 25(Oh)D (Vitamin D) Status in Swine Flu (H1N1)
Abstract
Background
Since swine flu has been declared pandemic in 2009 it has become a major challenging public-health problem associated with high morbidity and mortality. 25(OH)D deficiency is also pandemic and has been reported to be clinically correlated with decreased immunity and respiratory infections. The possible role of vitamin D in infections is implied from its impact on the innate and adaptive immune responses. This study is planned to evaluate clinical significance of 25(OH)D status on course and outcome in hospitalized cases of swine flu and to compare it with normal healthy subjects living in the same vicinity to evaluate if vitamin D is having any protective effect.
Material & Methods
Present prospective cross-sectional study was conducted on 79 RT-PCR confirmed cases of swine flu admitted during recent epidemic. All patients were evaluated thoroughly by clinical history physical examination and laboratory investigations as per Performa and followed-up during hospital stay. 25-hydroxyvitamin D (25(OH)D) estimation was done by electro-chemiluminescent Assay in all the cases and it was also done in 36 normal healthy family members of study patients living in the same vicinity (control group).
Results
High prevalence (70.9%) of low (≤30ng/ml) status of 25(OH)D was observed in cases of swine flu as compared to control group. 25(OH)D status was associated with severity of illness. Mean value of 25(OH)D in mechanically ventilated patients was 9.81±6.43 while it was 22.76±11.35 ng/ml in patients who do not required ventilation (p<0.05). Mean 25(OH)D level in patients who stayed in hospital for <5 days was 28.60±8.79 ng/ml, 24.18±11.67 for 6-10 days and 8.23±2.12 for >10 days (p<0.01). Mean value of 25(OH)D in patients who died was 9.59±5.90 ng/ml as compared to 23.13±11.62 ng/ml who survived (p<0.01).
Conclusion
Our study suggests that 25(OH)D may have preventive role for swine flu infection. Low level of 25(OH)D is associated with high morbidity in terms of increase requirement for mechanical ventilation, multiorgan dysfunction and long duration of hospital stay. 25(OH)D deficiency is associated with high mortality in swine flu. 25(OH)D status should be given due consideration in high risk patients especially during winter season.
Author Contributions
Academic Editor: Ishan Wadi, National Institute of Malaria Research, India.
Checked for plagiarism: Yes
Review by: Single-blind
Copyright © 2020 Bal Kishan Gupta, et al.
 This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Competing interests
The authors have no conflicts of interest to declare.
Citation:
Introduction
WHO declared swine flu (H1N1) pandemic on June 11, 2009 and since then it has become an important public health problem associated with repeated epidemics leading to high morbidity and mortality worldwide. Vitamin D deficiency is also pandemic.1,2 Vitamin D is best known for its influence on bone mineral density but now it is increasingly recognized for its extra-skeletal roles3, including an effect on inflammation and the immune response to infection.4,5 Vitamin D generates many extra-skeletal effects due to the vitamin D receptor (VDR) which is present in most tissues throughout the body. Vitamin D receptor (VDR) is a nuclear receptor for 1,25-Dihydroxyvitamin D3.6Cells involved in different biological processes such as immunity and inflammation have been found to possess VDR and are capable of metabolizing the active form of vitamin D suggesting that this nutrient may be an important factor in the immune response to infection.7,8
Recently anti‐viral effect of vitamin D has been described although underlying mechanism is poorly understood but finding suggestive of a complex interplay between viral infections and vitamin D, including the induction of anti‐viral state, functional immunoregulatory features, interaction with cellular and viral factors, induction of autophagy and apoptosis, and genetic and epigenetic alterations.9 Vitamin D has a modulatory and regulatory role in multiple processes, including host defence, inflammation, immunity, and epithelial repair. Patients with respiratory disease are frequently deficient in vitamin D.10,11Respiratory viral infections are common and are the main trigger of acute exacerbations and hospitalization in children and adults with asthma and other airways diseases.11 Respiratory monocytes/macrophages and epithelial cells constitutively express the vitamin D receptor. Vitamin D, acting through this receptor, may be important in protection against respiratory infections.12
1,25(OH)2D has been found to down-regulate pro-inflammatory cytokines such as IL-1, IL-6, IL-8, and TNFa.6 The anti-inflammatory effect of vitamin D is also carried by inhibition of NF-Kb. 1,25(OH)2D has been shown to promote the differentiation of monocytes into macrophages in mice and suppress the differentiation of human monocytes into dendritic cells.13 Because cathelicidin’s main mechanism of action is the destruction of envelope proteins of foreign agents, it may also be implicated in the destruction of influenza virus, which possesses an envelope.14 We do not find any study on vitamin D3 in swine flu patients therefore, this study was planned to evaluate 25(OH)D status and its clinical significance in cases of swine flu.
Material and Methods
Present prospective cross-sectional study was carried out on all the consecutive 79 confirmed cases of swine flu admitted in the dedicated swine flu ward, Department of Medicine, Sardar Patel Medical College & Associated Group of Hospitals, Bikaner during recent epidemic November 2018 to march 2019. Written informed consent was taken from all subjects or their legal guardians before enrolling for the study. Prior approval from Institutional Ethics Committee was taken.
Inclusion Criteria
All patients of swine flu confirmed by positive RT-PCR admitted in the dedicated swine flu ward.
Age >18 years.
Patient or their legal guardian giving consent to participate in the study.
Exclusion Criteria
1. Patients having influenza infection other than swine flu.
2. Patients or their legal guardian not giving informed consent
Swine flu was confirmed by RT-PCR. All patients were evaluated thoroughly by clinical history and physical examination as per Performa. Laboratory investigations were done in all patients at the time of admission including Complete blood count, Renal function test, Liver function test, Acid Base Gas analysis, Serum electrolytes (Na+, K+, Ca++), Serum 25-hydroxyvitamin D, X-ray Chest PA view and ECG. Other necessary special investigations like ultrasound, HRCT scan, MRI etc were done as per requirement. All patients were treated as per WHO guidelines15 with oseltamavir and supportive treatment and followed up during hospital stay as per protocol.
25-hydroxyvitamin D (25(OH)D) estimation was done by electro-chemiluminescent Assay16 using Elecsys 25(OH)D Assay (k060755) manufactured by Roche Diagnostics, Germany. Levels of 25(OH)D were classified into three categories as per US Endocrine Society (2011)17 criteria:
Deficient: - ≤20 ng/ml,
Insufficient: - 21-30 ng/ml and
Sufficient: - >30 ng/ml.
In our study low level of 25(OH)D (Hypovitaminosis D) means levels ≤30 ng/ml, and severe deficiency means ≤10 ng/ml.
Severity of swine flu was categorized as follow (according to Ministry of Health & Family Welfare, Govt of India guidelines revised on 18/10/2016): -
Category-A: Patients with mild fever plus cough / sore throat with or without body ache, headache, diarrhea and vomiting.
Category-B: It is further divided into B1 and B2. B1:-In addition to all the signs and symptoms mentioned under Category-A, these patient has high grade fever and severe sore throat. B2:- In addition to all the signs and symptoms mentioned under Category-A, individuals having one or more of the following high risk conditions (i) Children with mild illness but with predisposing risk factors (ii) Pregnant women (iii) Persons aged 65 years or older (iv) Patients with lung diseases, heart disease, liver disease kidney disease, blood disorders, diabetes, neurological disorders, cancer and HIV/AIDS (v) Patients on long term cortisone therapy.
Category-C: In addition to the above signs and symptoms of Category-A and B, if the patient has one or more of the following: Breathlessness, chest pain, drowsiness, fall in blood pressure, sputum mixed with blood, bluish discoloration of nails and worsening of underlying chronic conditions.
In addition, 25(OH)D estimation was also done in 36 normal healthy family members living in the same vicinity (control group) and compared it with swine flu patients (study group) to evaluate if vitamin D is having any protective effect.
Statistical Analysis
Anonymised data were analysed using SPSS software version 17.0. Chi square test, ANOVA test and Student ‘t’ test. Pearson’s Correlation test was done to evaluate correlation between 25(OH)D level and outcome and Multiple Linear Regression Analysis was done to predict outcome in relation to various variables. Correlation of 25(OH)D with hospital stay was analysed by R square value.
Results
Present study included a total of 79 cases out of which 35 (44.3%) were males (mean age 52.89±17.7 ranging 19-92 years) and 44 (55.7%) females (mean age 47.36±16.79 ranging 21 to 79 years) and they are compared with 36 normal healthy subjects (21 male, mean age 41.76±11.0 ranging 25-58 years and 15 female, mean age 38.27±12.1 ranging 18-63 years) of control group who were the family members of 23 patients of the study group living in the same vicinity and exposed to same environment and risk of getting swine flu infection. 49 Cases (62%) belonged to category C (mean age 54.02±13.14 years; age group wise 18-35= 7 cases, 14.28%, 36-55 = 17 cases, 34.69%, >55= 25 cases, 51.02%) and 30 were in category B (mean age 42.9±18.93 years; age group wise 18-35= 13 cases, 43.3%, 36-55 = 10 cases, 33.3%, >55= 7 cases, 23.3%) according to severity of swine flu, thus we found senior patients suffered more commonly with severe swine flu (p=0.006). 53 patients had one or more comorbidity (Chronic Obstructive Pulmonary Disease, Diabetes Mellitus, Ischemic Heart Disease, Interstitial lung disease, Hypertension, Bronchial Asthma, Hypothyroidism, Tuberculosis, congestive heart failure and Pregnancy) while 26 cases did not have any comorbidity.
25(OH)D status in relation to different epidemiological and clinical parameters in swine flu is shown in Table 1. Hypovitaminosis D (≤30ng/ml) status was seen in 70.9% of the cases and 44.4% of control group. Mean value of 25(OH)D in swine flu patients (study group) was 21.93±11.86 ng/ml ranging from 3.16-48.99 ng/ml and it was 31.32±8.84 ng/ml in control group ranging from 15.78- 58.3 ng/ml (p<0.00004). 10% of the cases (8 patients) had severe deficiency of vitamin D. Although there was no statistically significant difference in 25(OH)D level with regards to different age group but significant number of patients belonging to 18-35 and >55 years of age group were having hypovitaminosis D (80% and 78.2% respectively). Similarly, there was no difference of 25(OH)D level statistically between males and females but hypovitaminosis D was found more commonly in males (80%) as compare to females (63.6%). 75.6% of the cases belonging to urban area were having low status of 25(OH)D as compare to only 65.8% from rural area but statistically not significant. 25(OH)D levels were also statistically not significant between different category of BMI.
Table 1. 25(OH)D status in relation to different epidemiological and clinical parameters in swine flu| Parameter | No of cases (%) | 25(OH)D Status | p | ||||
| ≤20 | >20-≤30 | >30 | Low status ≤30 | Mean±SD | |||
| 25(OH)D | 0.00004* | ||||||
| Patients | 79(100) | 36(45.6) | 20(25.3) | 23(29.1) | 56(70.9) | 21.93±11.86 | |
| Control | 36(100) | 4(11.1) | 12(33.3) | 20(55.6) | 16(44.4) | 31.32±8.84 | |
| Age | 0.241 | ||||||
| 18-35 | 20(25.3) | 10(50) | 6(30) | 4(20) | 16(80) | 20.19±12.47 | |
| 36-55 | 27(34.2) | 11(40.7) | 4(14.8) | 12(44.4) | 15(55.5) | 25.06±12.71 | |
| >55 | 32(40.5) | 15(46.9) | 10(31.3) | 7(21.9) | 25(78.2) | 20.37±10.47 | |
| Sex Female | 35(44.3) | 19 (54.3) | 9(35.7) | 7(20) | 28(80) | 19.62±10.69 | 0.122 |
| 44(55.7) | 17(38.6) | 11(25) | 16(36.4) | 28(63.6) | 23.77±12.3 | ||
| Swine Flu Category | 0.037* | ||||||
| B | 31(39.2) | 9(29) | 9(29) | 13(42) | 18(58) | 25.47±11.33 | |
| C | 48(60.8) | 27(56.3) | 11(22.9) | 10(20.8) | 38(79.2) | 19.76±11.76 | |
| Co-morbidity | 0.267 | ||||||
| Yes | 53(67.1) | 26(49.1) | 14(26.4) | 13(16.5) | 40(75.5) | 20.89±12.28 | |
| No | 26(32.9) | 10(38.5) | 6(23) | 10(38.5) | 16(61.5) | 24.05±10.88 | |
| Residence Rural | 38(48.1) | 15(39.5) | 10(26.3) | 13(34.2) | 25(65.8) | 24.06±12.39 | 0.124 |
| Urban | 41(51.9) | 21(51.2) | 10(24.4) | 10(24.4) | 31(75.6) | 19.95±11.12 | |
| BMI | 5(6.3) | 2(40) | 1(20) | 2(40) | 3(60) | 26.85±14.86 | 0.155 |
| ≤18.518.51-24.99 | 46(58.2) | 22(47.8) | 14(30.4) | 10(21.7) | 36(78.3) | 20.33±11.45 | |
| 25-29.99 | 21(26.3) | 11(52.3) | 3(14.3) | 7(33.3) | 14(66.7) | 21.48±11.59 | |
| >30 | 7(8.9) | 1(14.3) | 2(28.6) | 4(57.1) | 3(42.9) | 30.31±11.25 | |
| SpO2 | 0.553 | ||||||
| ≤93 | 35(44.3) | 18(51.4) | 9(25.7) | 8(22.9) | 27(77.1) | 20.78±12.71 | |
| >93 | 44(55.7) | 19(43.2) | 11(25) | 14(31.8) | 30(68.2) | 22.38±11.08 | |
| Mechanical ventilation | 0.03* | ||||||
| Invasive | 6((7.6)) | 6(100) | 0 | 0 | 6(100) | 9.81±6.43 | |
| Non-Invasive | 10(12.7) | 4(40) | 3(30) | 3(30) | 7(70) | 21.99±14.62 | |
| Not required | 63(79.7) | 27(42.9) | 16(25.4) | 20(31.7) | 43(68.3) | 22.76±11.35 | |
| Hospital stay | 0.004* | ||||||
| ≤5days | 11(17.5) | 1(9.1) | 5(45.5) | 5(45.5) | 6(54.6) | 28.60±8.79 | |
| 6-10days | 37(58.7) | 15(40.5) | 8(21.6) | 14(37.8) | 23(62.2) | 24.18±11.67 | |
| >10days | 15(23.8) | 11(73.3) | 3(20) | 1(6.7) | 14(93.3) | 8.23±2.12 | |
| Outcome Survived | 72(91.1) | 30(41.7) | 19(26.4) | 23(31.9) | 49(68.1) | 23.13±11.62 | 0.003* |
| Died | 7(8.9) | 6(85.7) | 1(14.3) | 0 | 7(100) | 9.59±5.9 | |
25(OH)D was low in 79.2% patients with category C as compared to 58% of category B patients. A total of 56 cases of swine flu had hypovitaminosis D out of which 68% presented with category C symptoms while 32% presented with category B symptoms; while 23 patients of swine flu had sufficient level of 25(OH)D out of 57% presented with category B symptoms and 43% presented with category C symptoms. Mean value of 25(OH)D in category C patients was 19.76±11.76 ng/ml as compared to 25.47±11.33 ng/ml in patient presented with category B (p<0.05). 77.1% of the patients with SpO2 ≤93 at the time of hospitalization had hypovitaminosis D (14.3% had severe 25(OH)D deficiency) as compare to 68.2% with SpO2 >93 (6.8% had severe deficiency). More number of patients with comorbidity (specially with hypothyroidism, hypertension and diabetes) had hypovitaminosis D as compare to no-comorbidity although statistically not significant. (Figure 1)
Figure 1.showing correlation between 25(OH)D status with comorbidity in cases of swine flu
Correlation of 25(OH)D status with chest X-Ray finding at the time of hospitalization is shown in Table 2. hypovitaminosis D was associated with both the cases of bilateral patchy consolidation and whole right lung consolidation while 81.8% with right lower lobe consolidation and 80% with bilateral hilar prominence had hypovitaminosis D.
Table 2. Correlation of 25(OH)D status with different chest X-Ray findings| Chest X-ray Findings | Total (n=79) | Vitamin D3 Group | ||||||||
| <20 | 20-30 | >30 | <30 | |||||||
| No. | % | No. | % | No. | % | No. | % | No. | % | |
| B/L Hilar Prominence | 5 | 6.3 | 3 | 60.0 | 1 | 20.0 | 1 | 20.0 | 4 | 80.0 |
| B/L Lower Lobe Consolidation | 34 | 43.0 | 15 | 44.1 | 9 | 26.5 | 10 | 29.4 | 24 | 70.6 |
| B/L Patchy Consolidation | 1 | 1.3 | 1 | 100 | 0 | - | 0 | - | 1 | 100 |
| Left Lower Lobe Consolidation | 13 | 16.5 | 5 | 38.4 | 4 | 30.8 | 4 | 30.8 | 9 | 69.2 |
| Left Middle Lobe Consolidation | 5 | 6.3 | 2 | 40.0 | 1 | 20.0 | 2 | 40.0 | 3 | 60.0 |
| Right Lower Lobe Consolidation | 11 | 13.9 | 6 | 54.5 | 3 | 27.3 | 2 | 18.2 | 9 | 81.8 |
| Right Lung Consolidation | 1 | 13 | 1 | 100 | 0 | - | 0 | - | 1 | 100 |
| Right Middle Lobe Consolidation | 9 | 11.4 | 3 | 33.4 | 2 | 22.2 | 4 | 44.4 | 5 | 55.6 |
(Table 3) shows Correlation of 25(OH)D status with different laboratory parameters. We observed hypovitaminosis was associated with multi-organ dysfunction especially renal. High CRP titre was also corelated with hypovitaminosis D.
Table 3. Correlation of 25(OH)D status with different laboratory parameters in swine flu| Parameter | No of cases (%) | 25(OH)D Status | p | ||||
| ≤20 | >20-≤30 | >30 | Low status ≤30 | Mean±SD | |||
| Haemoglobin | 0.0645 | ||||||
| <10 | 30(38) | 18(60) | 7(23.3) | 5(16.7) | 25(83.3) | 19.61±9.67 | |
| ≥10 | 49(62) | 18(36.7) | 13(26.5) | 18(36.7) | 31(63.3) | 24.22±11.13 | |
| TLC | 0.6441 | ||||||
| <4000 | 40(50.6) | 22(55) | 10(25) | 8(20) | 32(80) | 19.65±10.65 | |
| 4000-11000 | 32(40.5) | 16(50) | 3(9.4) | 13(40.6) | 19(59.4) | 22.02±11.90 | |
| >11000 | 7(8.9) | 3(42.9) | 4(57.1) | 0 | 7(100) | 19.85±5.39 | |
| Platelet count | 0.3829 | ||||||
| <1.5 lakh | 24(30.4) | 12(50) | 7(29.2) | 5(20.8) | 19(79.2) | 20.15±11.81 | |
| >1.5 lakh | 55(69.6) | 24(43.6) | 13(23.6) | 18(32.7) | 37(67.3) | 22.70±11.79 | |
| CRP | 0.0395* | ||||||
| ≤5 | 11(13.9) | 3(27.3) | 3(27.3) | 5(45.5) | 6(54.5) | 26.17±10.34 | |
| >5 | 68(86.1) | 33(48.5) | 17(25) | 18(26.5) | 50(73.5) | 19.73±9.32 | |
| Blood Urea | 0.6207 | ||||||
| ≤40 | 50(63.3) | 24(48) | 10(20) | 16(32) | 34(68) | 22.44±12.54 | |
| >40 | 29(36.7) | 12(41.4) | 10(34.5) | 7(24.1) | 22(75.9) | 21.05±10.73 | |
| Serum Creatinine | 0.0132* | ||||||
| ≤1.5 | 64(81) | 26(40.6) | 16(25) | 22(34.4) | 42(65.6) | 23.33±11.93 | |
| >1.5 | 15(19) | 10(66.7) | 4(26.7) | 1(6.7) | 14(93.3) | 15.23±6.38 | |
| Serum Bilirubin | 0.0872 | ||||||
| ≤1.5 | 72(91.1) | 31(43.1) | 18(25) | 23(31.9) | 49(68.1) | 22.63±12.04 | |
| >1.5 | 7(9.9) | 5(71.4) | 2(28.6) | 0 | 7(100) | 14.64±4.9 | |
| SGOT | 0.5891 | ||||||
| ≤40 | 41(51.9) | 20(48.8) | 9(22) | 12(29.3) | 29(70.7) | 22.69±11.23 | |
| >40 | 38(48.1) | 16(42.1) | 11(28,9) | 11(28.9) | 27(71.1) | 21.23±12.51 | |
| SGPT | 0.0669 | ||||||
| ≤40 | 41(51.9) | 16(39) | 10(24.4) | 15(36.6) | 26(63.4) | 24.28±12.29 | |
| >40 | 38(48.1) | 20(52.6) | 10(26.3) | 8(21.1) | 30(78.9) | 19.39±10.98 | |
16 (20.3%) patients required mechanical support for ventilation out of them 6 (7.6%) patients who required invasive ventilator all of them (100%) were deficient in 25(OH)D while 10 (12.7%) required non-invasive ventilator out of which 70% were having hypovitaminosis D. Low status of 25(OH)D was observed in 81.3% of the cases who required mechanical support for ventilation as compared to 68.3% out of 63 (79.7%) cases who did not required mechanical ventilator.
Course of illness during hospitalization in patients (63 cases, 79.7%) who survived and were not put on ventilator was also corelated with 25(OH)D status. We observed that patient with low status of 25(OH)D required longer duration for recovery. Mean level of 25(OH)D in patients who stayed longer in hospital for >10 days was 8.23±2.12 as compare to 24.18±11.67 for 6-10 days stay and 28.60±8.79 ng/ml for <5 days stay (p<0.01). 25(OH)D level in relation to hospital stay for recovery in such patients shows inverse linear relationship (Correlation coefficient, R=0.9863; Correlation of determinant, R squared, R2= 0.9729) (Figure 2).
Figure 2.showing CORRELATION OF 25(OH)D LEVEL AND DURATION OF HOSPITAL STAY IN SURVIVED NON-VENTILATED PATIENTS OF SWINE FLU
In our study 7 patients died (mortality 8.9%) and all of them were having low levels of 25(OH)D. Mean value of 25(OH)D in patients who died was 9.59±5.90 ng/ml as compared to 23.13±11.62 ng/ml who survived (p<0.01). details of patients died of swine flu is shown in Table 4. Pearson's correlation coefficient analysis shows hypovitaminosis D is associated with poor outcome (Table 5, Figure 3). Multiple linear regression analysis indicates 25(OH)D is an independent prognostic predictor of outcome in cases of swine flu (Table 6, Figure 4).
Figure 3.showing Pearson’s Correlation analysis of outcome in relation to different parameters
Figure 4.showing Multiple Linear Regression Analysis (ANOVA) of various parameters in relation to outcome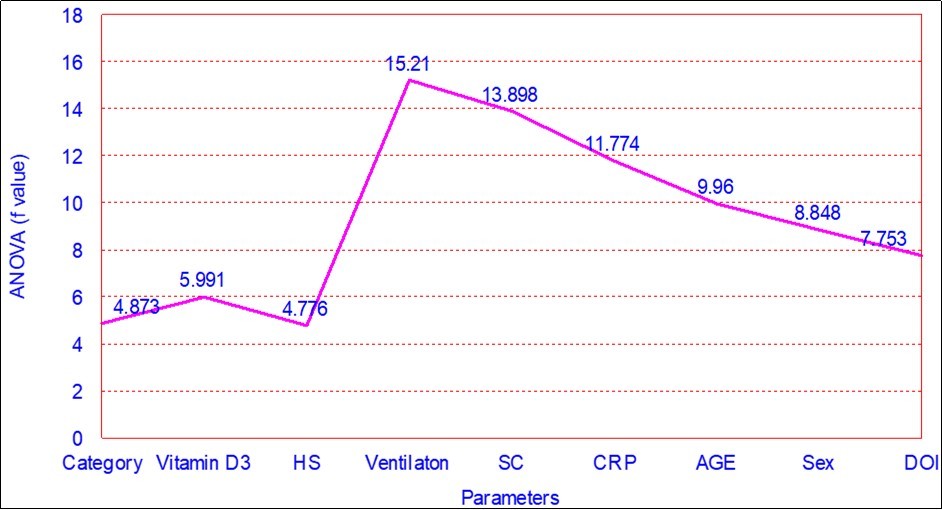
| Died Patient | |||||||
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | |
| Age | 70 | 32 | 87 | 25 | 60 | 50 | 60 |
| Gender | Female | Female | Male | Female | Male | Female | Male |
| Duration of Illness (days) | 5 | 3 | 6 | 3 | 3 | 3 | 5 |
| hospital stay (days) | 12 | 12 | 12 | 14 | 13 | 12 | 9 |
| Category | C | C | C | C | C | C | C |
| Comorbidity | AF | No | COPD | Pregnancy | IHD | DM+CHF | COPD |
| X-ray Finding | B/L Lobe Consolidation | B/L Lower Lobe consolidation | B/L Lower Lobe Consolidation | B/L Hilar Prominence | Lt Lower Lobe Consolidation | Rt Lower Lobe Consolidation | B/L Lowe Lobe Consolidation |
| 25(OH)D | 9.96 | 3.90 | 21.08 | 3.16 | 10.50 | 8.27 | 10.26 |
| Mechanical Ventilation | Invasive | Invasive | Invasive | Invasive | Invasive | Non-Invasive | Invasive |
| Parameters | r value | p value |
| Category | 0.244 | 0.030* |
| Age | 0.090 | 0.430 |
| Hospital Stay | 0.315 | 0.005* |
| Blood Urea | 0.335 | 0.003* |
| Serum Creatinine | 0.241 | 0.033* |
| Vitamin D3 | 0.327 | 0.003* |
| Duration of Illness | 0.084 | 0.464 |
| Ventilation | 0.619 | <0.001** |
| Coefficients a | ||||||
|---|---|---|---|---|---|---|
| Model | Unstandardized Coefficients | Standardized Coefficients | t | Sig. | ||
| B | Std. Error | Beta | ||||
| 1 | (Constant) | 2.143 | .109 | 19.578 | .000** | |
| category | -.143 | .065 | -.244 | -2.208 | .030* | |
| 2 | (Constant) | 1.929 | .134 | 14.394 | .000** | |
| category | -.104 | .064 | -.177 | -1.613 | .111 | |
| vitaminD3 | .007 | .003 | .285 | 2.597 | .011* | |
| 3 | (Constant) | 2.095 | .175 | 11.990 | .000** | |
| category | -.091 | .064 | -.156 | -1.422 | .159 | |
| vitaminD3 | .005 | .003 | .200 | 1.626 | .108 | |
| hospital stay | -.016 | .011 | -.181 | -1.470 | .146 | |
| 4 | (Constant) | .835 | .246 | 3.387 | .001* | |
| category | .005 | .055 | .009 | .093 | .926 | |
| vitaminD3 | .007 | .002 | .302 | 2.975 | .004* | |
| hospital stay | .011 | .010 | .126 | 1.129 | .262 | |
| ventilation | .451 | .072 | .637 | 6.262 | .000** | |
| 5 | (Constant) | .854 | .240 | 3.559 | .001* | |
| category | .015 | .053 | .025 | .276 | .783 | |
| vitaminD3 | .008 | .002 | .320 | 3.230 | .002* | |
| hospital stay | .015 | .010 | .164 | 1.495 | .139 | |
| ventilation | .450 | .070 | .637 | 6.432 | .000** | |
| serum creatinine | -.058 | .026 | -.195 | -2.280 | .026* | |
| 6 | (Constant) | .920 | .248 | 3.708 | .000* | |
| category | .025 | .054 | .042 | .458 | .648 | |
| vitaminD3 | .008 | .002 | .312 | 3.141 | .002* | |
| hospital stay | .013 | .010 | .144 | 1.294 | .200 | |
| ventilation | .426 | .074 | .603 | 5.793 | .000** | |
| serum creatinine | -.052 | .026 | -.174 | -1.986 | .051 | |
| Serum CRP | .000 | .000 | -.098 | -1.039 | .302 | |
| 7 | (Constant) | .918 | .250 | 3.669 | .000 | |
| category | .027 | .057 | .047 | .483 | .631 | |
| vitaminD3 | .008 | .002 | .315 | 3.096 | .003* | |
| hospital stay | .013 | .010 | .147 | 1.295 | .199 | |
| ventilation | .429 | .075 | .606 | 5.688 | .000** | |
| serum creatinine | -.050 | .028 | -.168 | -1.770 | .081 | |
| Serum CRP | .000 | .000 | -.096 | -.997 | .322 | |
| age | .000 | .002 | -.016 | -.164 | .870 | |
| 8 | (Constant) | .895 | .251 | 3.565 | .001* | |
| category | .019 | .057 | .032 | .326 | .746 | |
| vitaminD3 | .008 | .002 | .332 | 3.221 | .002* | |
| hospital stay | .013 | .010 | .150 | 1.324 | .190 | |
| ventilation | .404 | .079 | .571 | 5.099 | .000* | |
| serum creatinine | -.061 | .030 | -.203 | -2.008 | .048 | |
| Serum CRP | -.001 | .000 | -.131 | -1.287 | .202 | |
| age | .000 | .002 | -.007 | -.073 | .942 | |
| sex | .059 | .058 | .104 | 1.016 | .313 | |
| 9 | (Constant) | .893 | .271 | 3.289 | .002 | |
| category | .019 | .058 | .032 | .324 | .747 | |
| vitaminD3 | .008 | .003 | .333 | 3.165 | .002* | |
| hospital stay | .013 | .010 | .151 | 1.303 | .197 | |
| ventilation | .404 | .081 | .572 | 5.019 | .000** | |
| serum creatinine | -.061 | .030 | -.203 | -1.993 | .050 | |
| serum CRP | -.001 | .000 | -.131 | -1.271 | .208 | |
| age | .000 | .002 | -.008 | -.077 | .939 | |
| sex | .059 | .059 | .104 | 1.009 | .317 | |
| duration of disease | .000 | .018 | .002 | .027 | .978 | |
| a. Dependent Variable: outcome | ||||||
Discussion
Our study shows high prevalence of hypovitaminosis D in cases of swine flu as compared to normal healthy subjects living in the same vicinity of the patients ((70.9% versus 44.4%). Vitamin D deficiency is increasingly recognized as pandemic but its clinical significance is yet to be established properly and therefore it is mostly under treated in most part of the world18,19,20. The prevalence of 25(OH)D deficiency has been reported to vary from 33% to 95% from all over the world.18,19 We did not find any study in the world literature done on 25(OH)D in cases of swine flu, although previous studies have reported increase prevalence of 25(OH)D deficiency in cases of respiratory tract infection as Gindeet al concluded an increased prevalence of upper respiratory tract infection among subgroups with low 25(OH)D levels.11 Berry et al used cross-sectional data from 6789 participants in a nationwide 1958 British birth cohort and found linear association between 25(OH)D status and seasonal infections and concluded that each 10 ng/ml increase in 25(OH)D was associated with a 7% lower risk of infection.21 25(OH)D could be acknowledged as a “seasonal stimulus”, following R. Edgar Hope-Simpson, the British practitioner and self-educated epidemiologist, as he hypothesized that solar radiation produces a “seasonal stimulus” with an impact on the human immune system whose substrate levels are low during the influenza season, but peak when influenza is rare.22,23 Cholecalciferol is a prohormone normally made in the skin during sunny days when UVB radiations triggers the conversion of 7 dehydrocholesterol in the skin to 25(OH)D.24 25(OH)D hinders immune cell differentiation, restrains macrophage and monocyte interaction, and down regulates lymphocyte activity.25 In several inflammatory diseases, higher serum 25(OH)D levels or vitamin D supplementation has been associated with reduced levels of C-reactive protein, erythrocyte sedimentation rate and inflammatory cytokines.26 Vitamin D also enhances the function of the innate immune system by stimulating formation of the macrophage associated cathelicidin antimicrobial peptide.27 The effects of 1,25(OH)2D are mediated by it binding to the vitamin D receptor (VDR). VDR is a nuclear receptor and once it binds its ligand, VDR dimerizes with an isoform of the retinoid X receptor. These VDR-RXR heterodimers bind to vitamin D response elements present on target genes.28,29,30Thus low level of 25(OH)D in our cases may suggest its role in the pathogenesis of swine flu.
Our study shows that subjects living in the same vicinity of swine flu cases despite exposure does not developed disease as they were having optimal 25(OH)D status indicating protective role of 25(OH)D in the development of swine flu disease. Previous studies have shown role of 25(OH)D in acute respiratory tract infection as Karatekin et al showed in a group of new born admitted for acute lower respiratory infection, the 25(OH)D level was significantly lower (9 ng/mL) when compared to controls (16 ng/mL).31 Wayse et al showed that children with serum 25(OH)D levels greater than 9 ng/ml had significantly less risk for developing acute lower respiratory tract infection.32 Urashima et al did a clinical trial and concluded that 25(OH)D supplementation during winter reduces the incidence of influenza A.33
Our study shows status of 25(OH)D is correlated with severity of swine flu, most of the cases of category C were having low 25(OH)D as compared to category B. 25(OH)D metabolites have significant pleiotropic effects on innate and adaptive immune system cells as its deficiency affects the development of sepsis with several mechanism that includes adaptive and innate immune modulation suppression of inflammatory response increased cathelicidins and defensins.34 Olejarova et al showed that patients with septic shock had a lower level of vitamin D compared with septic patients without organ dysfunction and concluded that 25(OH)D deficiency is associated with severity of sepsis.35 Although pathophysiology of MODS remains incompletely understood, it arises through the interactions of insulting agent like infective organism and host inflammatory response. As vitamin D deficiency is associated with dysregulated immune and inflammatory response, it may be a contributing factor for development of multi-organ dysfunction along with other factors like virulence of infectious agent, therefore, presence of vitamin D deficiency may be considered as a sign of multi-organ dysfunction. We also observed multiorgan dysfunction in our cases of swine flu associated with 25(OH)D deficiency as evaluated by laboratory parameters. This indicates that degree of 25(OH)D deficiency may also be related with severity of swine flu disease. Correlation between degree of vitamin D deficiency with severity of the illness and poor outcome has been reported previously in other diseases.11,36,37
Bilateral or unilateral lower lobe involvement was the commonest radiological findings in our cases, similar to reported by previous workers.38,39 We observed low levels of 25(OH)D in such cases were associated with high mortality.
Our study shows that patient with 25(OH)D deficiency are more likely to develop respiratory failure and require mechanical support as compared to patient with normal 25(OH)D status. 27.8% of the cases (10 out of 36) with vitamin D deficiency required mechanical respiratory support as compare to none with normal levels. 25(OH)D deficiency has been shown to be associated with an increased risk of intensive care admission in patients with pneumonia, development of ARDS and mechanistically related to exaggerated lung alveolar inflammation and alveolar epithelial cell injury, hypoxia, respiratory failure and requirement of ventilation.40,41
When we correlated 25(OH)D level with course of illness during hospitalization in patients who survived and were not put on ventilator we found that 25(OH)D level have linear relationship with duration of hospital stay for recovery as shown in Figure 2, lower the 25(OH)D level longer the hospital stay. Han et al showed in a clinical trial that there was a significant decrease in hospital length of stay over time in the patient supplemented with 250,000 IU and the 500,000 IU 25(OH)D group, compared to the placebo group (25 ± 14 and 18 ± 11 days compared to 36 ± 19 days, respectively; p<0.05).42 Other workers also concluded that 25(OH)D level had a significant inverse correlation with length of hospital stay.36,37,43
Our study also shows that 25(OH)D deficiency was associated with high mortality. Gaksch et al meta-analysed 26916 study participants observed an association between low 25(OH)D level and increased risk of mortality.44 Garland et al concluded that Serum concentrations less than or equal to 30 ng/ml also were associated with higher all-cause mortality when compared with those greater than 30 ng/ml (P <0.01).45 Health et al after screening 722 unique records reported inverse associations between 25(OH)D concentration and all-cause mortality.46
I think disease occurrence may be the result of inappropriate systemic inflammatory response to any infection or any other non-infectious trigger and degree of inappropriate systemic inflammatory response may affect its course and outcome. Vitamin D may play an important role in harmonizing appropriate systemic inflammatory response. Although many clinical intervention trials has not shown benefit of vitamin D supplementation47may be becausesupplementation of vitamin D may not have an acute effect but long term maintenance of sufficient D3 status may be beneficial in optimising immunity level.
Conclusion
Our study suggests that hypovitaminosis D may increase susceptibility for swine flu infection. Low level of 25(OH)D is associated with high morbidity in terms of increase requirement for mechanical ventilation, multiorgan dysfunction and long duration of hospital stay. Patients with 25(OH)D deficiency are associated with high mortality in swine flu. 25(OH)D status should be given due consideration in high risk patients and especially during winter season.
Authors Contribution
Designed the study: BKG. Drafted the manuscript: BKG, KN and MLS. Approved the final version to be published: BKG. Carried out clinical assessment, data collection and review of literature: BKG, KN, RKB, JG and SR. Evaluated and analysed laboratory data and their interpretation: BKG, KN and MLS. All authors read and approved the final manuscript. Guarantors of the paper: BKG and KN.
Funding
None
Ethical Approval
A prior approval has been taken from the Institutional Ethics Committee to carry out this work, and an informed consent was obtained from the subjects enrolled in this study.
References
- 1.Cashman K D. (2020) . Vitamin D Deficiency: Defining, Prevalence, Causes, and Strategies of Addressing. Calcif Tissue Int;106(1): 14-29.
- 2.Roth D E, Abrams S A, Aloia J. (2018) Global prevalence and disease burden of vitamin D deficiency: a roadmap for action in low- and middle-income countries. , Ann N Y Acad 1430(1), 44-79.
- 4.Gunville C F, Mourani P M, Ginde A A. (2013) The Role of Vitamin D in Prevention and Treatment of Infection. Inflamm Allergy Drug Targets;. 12(4), 239-245.
- 5.Kempker J A, Han J E, Tangpricha V, Ziegler T R, Martin G S. (2012) Vitamin D and sepsis: An emerging relationship. , Dermato-Endocrinology; 4(2), 101-108.
- 7.Bikle D D. (2008) Vitamin D and the immune system: role in protection against bacterial infection. , Curr Opin Nephrol Hypertens; 17, 348-52.
- 8.Shirvani-Farsani Z, Behmanesh M. (2019) RNAi-mediated knockdown of VDR surprisingly suppresses cell growth in Jurkat T and U87-MG cells. , Heliyon; 5(11), 02837.
- 9.Teymoori-Rad M, Shokri F, Salimi V, Marashi S M. (2019) The interplay between vitamin D and viral infections. Rev Med Virol;. 29(2).
- 10.Gupta B K, Meena S L, Saini M, Nehara H R, Saini M L et al. (2016) Evaluation of vitamin D level in patients with chronic obstructive pulmonary disease (COPD) and its clinical correlation. , IJMHS; 3(2), 107-112.
- 11.Ginde A A, Mansbach J M, Camargo CA Jr. (2009) Association between serum 25-hydroxyvitamin D level and upper respiratory tract infection in the Third National Health and Nutrition Examination Survey. , Arch Intern Med; 169(4), 384-390.
- 12.Zdrenghea M T, Makrinioti H, Bagacean C, Bush A, Johnston S L et al. (2017) Vitamin D modulation of innate immune responses to respiratory viral infections. Rev Med Virol;. 27(1), 1-13.
- 13.Dixon B M, Barker T, McKinnon T, Cuomo J, Frei B et al. (2012) Positive correlation between circulating cathelicidin antimicrobial peptide (hCAP18/LL-37) and 25-hydroxyvitamin D levels in healthy adults. , BMC Res Notes; 5, 575.
- 14.Kempker J A, Martin G S. (2013) Vitamin D and Sepsis: From Associations to Causal Connections. Inflamm Allergy Drug Targets;. 12(4), 000.
- 15.WHO. (2010) WHO guidelines for pharmacological management of pandemic influenza A (H1N1) 2009 and other influenza viruses. Part I Recommendations. Revised.
- 16.Arneson W L, Arneson D L. (2013) Current methods for routine clinical laboratory testing of vitamin D levels. , Lab Med; 44(1), 38-42.
- 17.Holick M F, Binkley N C, Bischoff-Ferrari H A, Gordon C M, Hanley D A.Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. , J Clin Endocrinol Metab; 96, 1911-1930.
- 18.Holck M F, Chen T C. (2008) Vitamin D deficiency: a worldwide problem with health consequences. , Am J Clin Nutr; 87, 1080-6.
- 19.Suchanda G, Yadav K S, Gomes M W. (2016) Vitamin D status in Indian population. , Major Hlth Concern, World J Pahrmactucial Res; 5(1), 362-378.
- 20.Crowe F L, Jolly K, MacArthur C, Manaseki-Holland S, Gittoes N et al. (2019) Trends in the incidence of testing for vitamin D deficiency in primary care in the UK: a retrospective analysis of The Health Improvement Network (THIN). , BMJ Open; 9(6), 1-9.
- 21.Berry D J, Hesketh K, Power C, Hyppönen E. (2011) Vitamin D status has a linear association with seasonal infections and lung function in British adults. , Br. J. Nutr; 106, 1433-1440.
- 22.Cannell J J, Vieth R, Umhau J C, Holick M F, Grant W B et al. (2006) Epidemic influenza and vitamin D. Epidemiol Infect;. 134, 1129-40.
- 23.Hope-Simpson R. (1981) The role of season in the epidemiology of influenza. , Epidemiol. Infect; 86, 35-47.
- 24.Holick M F. (2006) High prevalence of vitamin D inadequacy and implications for health. , Mayo Clin Proced; 81, 297-299.
- 26.Mohan B D, Gordon S A, Cruz J, Cosman F, Contorna M T. (2003) Cytokine profile in patients with multiple sclerosis following vitamin D supplementation. , J Neuroimmunol; 134, 128-32.
- 27.Liu P T, Stenger S, Li H, Wenzel L, Tan B H et al. (2006) Toll-like receptor triggering of a vitamin D-mediated anti-microbial response. , Science; 311(5768), 1770-3.
- 28.Chawla A, Repa R, Evans M, Mangelsord D J. (2001) Nuclear receptors and lipid physiology: opening the X-files. , Science; 294, 1866-70.
- 30.Yasmin R, Williams R M, Xu M, Noy N. (2005) Nuclear import of the retinoid X receptor, the vitamin D receptor, and their mutual heterodimer. , J Biol Chem; 280(48), 40152-60.
- 31.Karatekin G, Kaya A, Salihoglu O, Balci H, Nuhoglu A. (2009) Association of subclinical vitamin D deficiency in newborns with acute lower respiratory infection and their mothers. , Eur J Clin Nutr; 63(4), 473-477.
- 32.Wayse V, Yousafzai A, Mogale K, Filteau S. (2004) Association of subclinical vitamin D deficiency with severe acute lower respiratory infection in Indian children under 5 y. , Eur J Clin Nutr; 58(4), 563-567.
- 33.Urashima M, Segawa T, Okazaki M, Kurihara M, Wada Y et al. (2010) Randomized trial of vitamin D supplementation to prevent seasonal influenza A in schoolchildren. , Am. J. Clin. Nutr; 91, 1255-1260.
- 34.Gul F, Arslantas M K, Bilgili B, Besir A, Kasapoglu U S et al. (2019) Serum vitamin D level variation in SIRS, sepsis and septic shock. , Shock; 32, 102-6.
- 35.Olejarova M, Dobisova A, Suchankova M, Tibenska E, Szaboova K et al. (2019) Vitamin D deficiency – a potential risk factor for sepsis development, correlation with inflammatory markers. SOFA score and higher early mortality risk in sepsis. Bratisl Lek Listy;. 120, 284-90.
- 36.Gupta B K, Saini M L, Nehara H R, Meena S L, Saini M et al. (2016) Evaluation of vitamin D deficiency in patients with chronic liver disease and its clinical significance. , IJN; 2(2), 29-35.
- 37.Gupta B K, Ranva A K, Meena S L, Sonkaria R K, Gupta J. (2018) Clinical evaluation of significance of 25(OH)D levels in patients with Organophosphorus poisoning. , IJN; 3(1), 16-29.
- 38.RCA Dancer, Parekh D, Lax S, D’Souza V, Zheng S et al. (2015) Vitamin D deficiency contributes directly to the acute respiratory distress syndrome (ARDS). , Thorax; 70, 617-24.
- 39.Panchal V D, Desai P, Vadel M K. (2015) Role of chest XRAY in assessing the severity in H1N1 Influenza cases. , NJMR; 5, 305-8.
- 40.Bakhshayeshkaram M, Saidi B, Tabarsi P, Soheila Zahirifard S, Ghofrani M. (2011) . Imaging Findings in Patients With H1N1 Influenza A Infection. Iran J Radiol; 8(4), 230-234.
- 41.Yadav S, Joshi P, Dahiya U, Baidya D K, Goswami R et al. (2018) Admission vitamin D status does not predict outcome of critically ill patients on mechanical ventilation: an observational pilot study. , Ind J Anaesth; 62(1), 47-52.
- 42.JL Han JE Jones, Tangpricha V, Brown M A, Hao L. (2016) High dose vitamin D administration in ventilated intensive care unit patients: A pilot double blind randomized controlled trial. , J Clin Translat Endocrin; 4, 59-65.
- 43.Botros R M, AbdElsalam Besibes MM, Bahaaeldin A M, Abo Elyazed S. (2018) Vitamin D status in hospitalization chronically ill patients. , J am Coll Nutr; 37(7), 578-82.
- 44.Gaksch M, Jorde R, Grimnes G, Joakimsen R, Schirmer H. (2017) Vitamin D and mortality: individual participant data meta-analysis of standardized 25 hydroxyvitamin D in 26916 individuals from a European consortium. PLoS One;. 12(1), 0170791.
- 45.Garland C F, Kim J J, Mohr S B, Gorham E D, Grant W B. (2014) Meta analysis of all cause mortality according to serum 25 hydroxyvitamin D. , Am J Pubic Hlth; 104(8), 43-50.
