Sulfonamides: Historical Discovery Development (Structure-Activity Relationship Notes)
Abstract
Sulfonamide group is a magic group introduced as the main core for different bio-activities in drug industry. According to its substitutes, literature divides sulfonamide derivatives to antibacterial sulfonamides and non-anti-bacterial sulfonamides. As Data was collected from different sources such as Drug Bank.com and Pubchem.com databases and then was analyzed, we found that these compounds are different in their pharmacokinetics and pharmacodynamics; in addition to their sulfa cross allergy property. We presented these differences from these compounds changes in their chemical structure, in a way to build a solid base that can be depended on for developing new drugs from these compounds that interact with different receptors.
Author Contributions
Academic Editor: Inder Kaur, Nottingham Trent university, UK
Checked for plagiarism: Yes
Review by: Single-blind
Copyright © 2018 Farah Yousef,et al
 This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Competing interests
The authors have declared that no competing interests exist.
Citation:
Introduction:
Sulfonamide derivatives medical groups’ discovery can be more similar to a string of distinguished pearls. They have in common the same main core but they differ in their bioactivities;1, 2The common core structure of sulfonamide is illustrated in Figure 1. Literature used to divide sulfonamides into anti-bacterial sulfonamides; with an aromatic amine, and non-anti-bacterial sulfonamides; without an aromatic amine.3 The last includes agents work as anti-inflammatory, anti-hyperglycemia, diuretics, serotonin antagonists, or other different pharmacology.2, 3 As we assume in this paper this activity depends on the substitutes that the compound chemical structure has in addition to sulfonamide group. We tried here to collect sulfonamide drugs properties and chemical structures to compare between them from structure differences that reflect on the activities they have. In other words we tried to set Structure-Activity-Relationship (SAR) from these chemical structures for sulfonamide core. This helps the researchers more in case they need a reference for these compounds collected in one paper.
Figure 1.Sulfonamide common core structure
Anti-Bacterial Sulfonamides:
Sulfonamide was firstly noted as anti-bacterial in 1900’s by Gerhard Domagk; a Nobel Prize winner in 1939. In his attempt to save his daughter from streptococci killing infection, he observed that prontosil; a sulfonamide dye, is able to selectively restrain the infectious bacteria cells. In 1936, Ernest Fourneau found out prontosil pathway in human body. He discovered that this dye was a pro-drug. It, actually changes in human body to sulfanilamide which is the anti-bacterial active agent.
This invention triggered the discoveries of other anti-bacterial members derived from this chemical group such as sulfapyridine in 1938 against pneumonia, and sulfacetamide in 1941 against urinary tract infections, and succinoylsulfathiazole in 1942 against gastrointestinal tract infections. Sulfathiazole was commonly used during World War II to cure soldier wounds’ infections. On the contrary, sulfanilamide was not very used due to its greater human toxicity. Later on, sulfisoxaide, sulfamethoxazole, sulfacetamide, mafenide and sulfadiazine silver were discovered, and those four agents are the sulfonamide anti-bacterial agents have been in the clinical use so far.
Sulfonamide anti-bacterial medications; also called sulfa drugs, are competitive inhibitors of p- amino benzoic acid in the folic acid metabolism cycle in the organisms . 4, 5 They have a common core structure shown in Figure 2.
Figure 2.Anti-bacterial Sulfonamide ‘s common core structure.
They can be classified as Oral absorbable, oral non-absorbable, and topical agents.4 Oral absorbable agents are also divided into short acting agents such as sulfisoxaide, medium acting agents such as sulfamethoxazole and long acting agents such as sulfasalazine.
Oral non absorbable agent group includes only sulfasalazine, while topical agents have sulfacetamide, mafanide, and silver sulfadiazine. Chemical structures of these groups are shown in Figure 3, and their properties are shown in Table 1. Sulfonamides that do not contain this aromatic amine group undergo different metabolic pathways. 6
Figure 3.Anti-bacterial Sulfonamide members’ structures.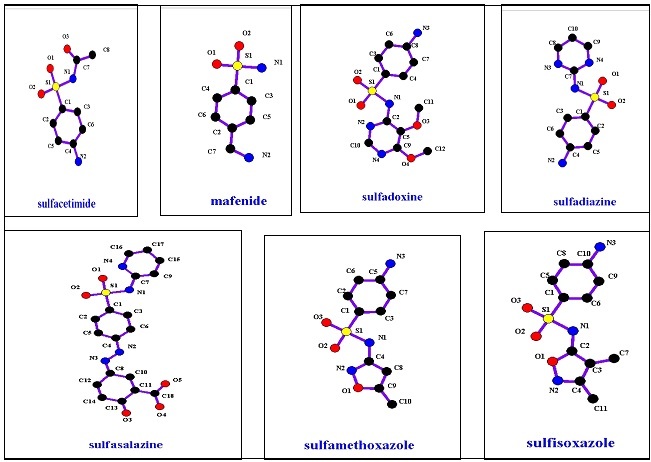
| Compound Name | Log P | Molecular Weight | T 1/2 |
|---|---|---|---|
| Sulfisoxazole | 1.01 | 267.303 | 6 |
| Sulfamethoxazole | 0.89 | 253.276 | 10 |
| Sulfadoxine | 0.7 | 310.328 | N\A |
| Sulfasalazine | 3.8 | 398.393 | 5- 10 |
| Sulfacetamide | -0.96 | 214.239 | 7-12.8 |
| Mafenide | N\A | 186.229 | N\A |
| Silver Sulfadiazine | N\A | 357.136 | N\A |
Non Anti-Bacterial Sulfonamide:
Anti-Hyperglycemic Agents:
This group of drugs is commonly used in type 2 diabetes treatment.7 These drugs' history goes back to 1937, when Ruiz made experiments on sulfa drugs.8, 9, 10 Later, in 1942, Janbon confirmed this efficacy when anti-bacteria sulfonamide; p-amino-sulfonamide-isopropylthiodiazole, caused such an efficacy as side effect in patients treated from typhoid. 11
Studies on sulfonamide bioactivities expanded when Laboratories proved that sulfa drugs stimulated beta cell release of insulin. 1 In 1950s, carbutamide; 1-butyl-3-sulfonylurea, was the first sulfonylurea compound presented in the clinical use for diabetes therapy , but not for too long as it had adverse effects on bone marrow.3
In 1956, Germany introduced tolbutamide; sulfa drugs derivative, as the first sulfonylurea compound to be in clinical use for diabetes treatment. Other first generation sulfonylurea compounds; acetohexamide, tolazamide, and chlorpropamide were available in the German market. 1, 2
Glyburide and glipizide; more potent sulfonylurea members entered the US drug market in 1984; after more than a decade of their usage in Europe. 12 Furthermore, glimipiride, the most potent sulfonylurea compound, was not commercially introduced till 1995 in the US drug market. 13
The mechanism of anti-hyperglycemic agents action is the increase of insulin hormone secretion from pancreatic beta cells.14, 15, 16 Therefore, they are considered inactive for dysfunctional pancreas. 17 Their main active site is in ATP sensitive potassium ion channels; Kir 6.2\SUR1; Potassium Inward Rectifier ion channel 6.2\ Sulfonylurea Receptor 1.
The common core structure of these compounds is presented in Figure 4. From this structure, it can be found that these sulfonylurea compounds are derived from sulfonamide (Figure 1) by replacing R1 with (-CO-NHR2) and R2 with H, NH2 with R3. R1, R2, R3 in sulfonylurea structure which are responsible for the different properties sulfonylurea compounds have. Figure 5 shows sulfonylurea family members and Table 2 shows their properties.
Figure 4.Sulfonylurea general structure.
Figure 5.Sulfonylurea anti-hyperglycemic agents’ structures.
| Compound Name | pK a | Log P | Molecular Weight | T 1\2 | |
|---|---|---|---|---|---|
| Tolbutamide | 5.16 | 2.3 | 270.347 | 7 | |
| Tolazamide | 3.6 | 2.69 | 311.4 | 7 | |
| Acetohexamide | 6.6 | 2.3 | 324.395 | N\A | |
| Carbutamide | N\A | 1.01 | 271.335 | N\A | |
| Chlorpropamide | 5.13 | 2.2 | 276.735 | 36 | |
| Glycyclamide | N\A | N\A | 296.119 | N\A | |
| Metahexamide | 3 | N\A | 311.4 | N\A | |
| Glyburide | N\A | 4.9 | 494.003 | 2-4 | |
| Gliclazide | N\A | 2.6 | 323.411 | 6-15 | |
| Glipizide | 5.9 | 1.91 | 445.538 | 3-5 | |
| Glibornurinde | N\A | N\A | 276.735 | N\A | |
| Gliquidone | N\A | 4.5 | 527.636 | N\A | |
| Glisoxepide | N\A | N\A | 449.526 | N\A | |
| Glyclopyramide | N\A | N\A | 303.761 | N\A | |
| Glymidine | 6.92 | 1.27 | 309.34 | 4 | |
| Glimiiride | N\A | 3.9 | 490.619 | 5 | |
It is worth mentioning that not all sulfonylurea derivatives are anti-hyperglycemic agents. Most of them are herbicides. 18 To eliminate the confusion about this point, it is important to present the common core structure of these sulfonylurea herbicides;6 (Figure 6), which shows the difference between them and anti-hyperglycemic agents derived from sulfonylurea.
Diuretics:
We all know that diuretics play an effective role in hypertension treatment.19 There are many pharmaceutical combinations between them and anti-hypertension agents.20 In general, diuretics such as carbonic anhydrase inhibitors, thiazides and loop diuretics are sulfonamide compounds. The chemical structures of these sub-group members are shown in Figure 7. Loop diuretics are considered safer and high ceiling diuretics. Their efficacy has linear relationship with their doses, to the contrary of thiazides which are low-ceiling diuretics.21, 22 These properties can be attributed to the reason that the loop diuretics are sulfonamide derivatives not thiazide ones.
Figure 7.carbonic anhydrase inhibitors’ structures.
Figure 8 shows the common core structure of thiazide diuretics, and Figure 9 presents thiazide family members. Loop diuretic common core structure is presented in Figure 10; where X can be N or C, while Figure 11 presents different members of it. Table 3 presents diuretic compounds’ properties.
Figure 8.Thiazide diuretics’ general structure.
Figure 9.Thiazides structures.
Figure 10.Loop diuretics’ general structure.
Figure 11.loop diuretics’ structures.
| Compound Name | pKa | Log P | Molecular Weight g\mol | T 1\2 (h) |
| Acetzolamide | 7.2 | -0.45 | 222.237 | 9 |
| Brinzolamide | N\A | -1.8 | 383.496 | 111 day |
| Dichlorophinamide | 7.4 | 0.2 | 305.144 | N\A |
| Dorzolamide | N\A | -1 | 324.428 | 4 months |
| Methazolamide | 7.3 | 0.13 | 236.264 | 14 h |
| Sulthiame | N\A | N\A | 290.352 | N\A |
| Metolazone | 9.72 | 2.5 | 365.832 | 14 h |
| Bendroflumethiazide | 8.5 | 1.19 | 421.409 | 8.5 |
| Chlorothiazide | 6.85 | -0.24 | 295.712 | 2 h |
| Chlortalidone | N\A | 0.85 | 338.762 | 40 h |
| Clopamide | N\A | N\A | 345.842 | N\A |
| Diazoxide | 8.74 | 1.2 | 230.666 | 28 h |
| Hydrochlorthiazide | 7.9 | -0.07 | 297.728 | 14.8 |
| Hydroflumethiazide | 8.9 | 0.36 | 331.284 | 27 h |
| Indapamide | 8.8 | 2.2 | 365.832 | 14 |
| Xipamide | N\A | N\A | 354.805 | N\A |
| methyclothiazide | 9.4 | 1.42 | 360.224 | N\A |
| Bumetanide | N\A | 2.6 | 364.416 | 60-90 min |
| Furosemide | N\A | 2.03 | 330.739 | 1.5 |
| Piretanide | N\A | 3.92 | 362.4 | N\A |
| torasemide | N\A | 2.3 | 348.421 | 3.5 |
Thiazide acts at the proximal part of the distal tubule. They interfere with Sodium transfers which increases excretion and urine volume. This results in a reduction of blood volume.23 These diuretics are well absorbed after oral administration, well distributed and undergo a hepatic metabolism. Since their effect target tissues are the kidney , renal failure decreases their efficacy. Thiazides must be taken in awareness with beta-blockers. Together are considered a high risk to cause diabetes in people with impaired glucose tolerance, features of the metabolic syndrome, or obesity.
Serotonin Antagonists:
Many sulfonamide compounds are 5-HT3 receptor antagonists. As a consequence, they work as anti depressants such as Naratriptan and Sumatriptan.23, Figure 12 shows chemical structures of these compounds, and Table 4 presents their properties.
Figure 12.Sulfonamide anti depressants’ structures.
| Compound Name | Log P | Molecular Weight g\mole | T 1\2 (h) |
| Sumatriptan | 1.6 | 335.466 | 5-8 |
| Naratriptan | 0.93 | 295.401 | 2.5 |
Anti– Inflammatory Agents:
Celecoxib, rofecoxib, and valdecoxib are sulfonamide derivative work as anti-inflammatory agents.24 Their mechanism of action is selectively inhibiting Cyclo-Oxygenase-2 Enzyme (COX-2 enzymes).25 This prevents prostaglandins and other inflammatory substrate production. Figure 13 illustrates their chemical structures. Table 5 presents their properties.
Figure 13.sulfonamide anti-inflammatory agents’ general structures.
| Compound Name | Log P | Molecular Weight g\mole | T 1\2 (h) |
| Celecoxib | 3.47 | 381.373 | 11 |
| Rofecoxib | 1.56 | 314.355 | 17 |
| Valdecoxib | 2.67 | 314.359 | 8-11 |
Other Pharmacological Sulfonamide Compounds:
These include protease inhibitors with activity against Human Immunodeficiency Virus Type 1 ( HIV-1) such as amprenavir and fosamprenavir,26,27 anti convulsant agent used in the treatment of epilepsy and migraine such as topiramate,28 anti hypertension as sotalol,29 anti-inflammatory and immunosuppressive agent with anti bacterial and antibiotic properties such as dapsone, anti-arrhythmia agent as Ibutilide, a uricosuric and renal tubular blocking agent; Probencid which is used to treat chronic gouty arthritis, and anti seizure such as zonisamide. These compound chemical structures are shown in Figure 14, and their properties are shown in Table 6.
Figure 14.sulfonamides with different pharmacologies agents’ structures.
| Compound Name | pKa | Log P | Molecular Weight g\mole | T 1\2 (h) |
| Amprenavir | ------- | 2.2 | 505.63 | 7.1-10.6 |
| Fosamprenavir | 1.7 | 2.2 | 585.609 | 7.7 |
| Dapsone | 2.41 | 0.97 | 248.3 | 28 |
| Ibutilde | -------- | 4.31 | 384.579 | 6 |
| Probencid | 3.4 | 3.21 | 285.358 | 6-12 |
| Sotalol | ------ | 0.24 | 272.363 | 12 |
| Zonisamide | 10.2 | 0.36 | 212.223 | 63 |
| Topiramate | ------- | -0.7 | 339.359 | 21 |
Structure Activity Relationship Notes:
Comparing the common core structures between the different groups of sulfonamides based on their bioactivity, we conclude to:
Anti-Bacterial agents: NH2 bounded to aromatic group is free with no bounded moieties. while R1 connected to NH2 in sulfonamide group could be H or any heterocyclic group.
Anti-hyperglycemic agents: Substituting aromatic NH2 with R1 ( this could be NH2 or Alkyl moiety). It also has sulfonylurea moiety instead of sulfonamide group where R2 connected to urea moiety could be Alkyl, Aromatic group, or heterocyclic group.
Herbicides: They also have sulfonylurea group, but it has R1 in Orto position instead of the aromatic NH2 which was in the para position. R2 moiety connected to sulfonylurea group is aromatic heterocyclic group.
When the sulfonamide group is free of moieties from NH2 side. While R1 connected to SO2 group differs between the pharmacologic groups as follows:
Carbonic anhydrase inhibitors: R1 is aromatic hetero cyclic group.
Thiazides: R1 is aromatic cycle, where Cl or F is in orto position. In para position, heterocyclic group or a moiety that has SO2 could bound.
Loop diuretics: R1 is aromatic cycle with groups in orto or para positions.
Serotonin ant-agonists: R1 is alkyl moiety connected to hetero cycle which might be aromatic or non-aromatic.
Anti-inflammatory agents: R1 is Aryl group where in para position there is heterocyclic group.
Sulfa Drug Cross-Allergy:
Studies have proved non-cross allergic reactivity among sulfa based structure drugs. In fact, allergy incidences toward these medications happen commonly in antibacterial sulfa drugs, but not in the other sulfa based compounds. 30
However, sulfonamide diuretics are not far from the risk of cross-reactivity of sulfonamide allergy. Patients who are allergic to other sulfonamides showed doubled allergic reactivity toward sulfonamide diuretics. 31
Conclusion:
This paper has presented a number of compounds that were derived from this unique chemical group with a variety of pharmacological effects that served human health. We consider sulfa drugs are a great discovery. One can develop chemical structure as potential drugs in the future by substituting R moieties or adding halogens or inserting any changes the researcher finds necessary in sulfonamide structure for his drug development. One can also have molecular modeling for one compound from different sub-activity groups to find out if they have any effect on the other compounds receptors in a way to develop new agents from the same chemical group.
References
- 1.Ayub Z, Ayub S, Shakoor M. (2013) Sulfa allergy: cross-reactivity versus multiple concurrent allergies, American journal of infectious diseases. 4, 148-154.
- 2.Branowska D, Fusiarz l. (2014) Biological activity and synthesis of sulfonamide derivates: a brief review, Chemik. 68(7), 620-628.
- 3.Schinnar R. (2003) Apter AJ, Absence of cross reactivity between sulfonamide antibiotics and sulfonamide non-antibiotics. , N. ENGL. J. MED 1628-1635.
- 4.Kishore D, Pareek A. (2013) A short review on sulphonamides, International journal of pharma and bio sciences. 4, 812-820.
- 5.Lesch J E. (2007) The first miracle drugs: how sulfa drugs transformed medicine. , New York: OxfordUniversityPress,x,364p. p
- 6.Healy R.(2007,6) Which diuretics are safe and effective for patients with a sulfa allergy?. , The Journal of Family 56(6), 488-490.
- 7.Uzor Ph, Patience O. (2015) Oral anti-diabetic agents –review and updates, Bristish journal of medicine and medical research. 5, 134-159.
- 8.Levine R.Sulfonylureas: Background and development of the field. Diabetes Care 1984; 7(Suppl 1):. 37.
- 9.Seltzer H. (1980) Efficacy and safety of oral hypoglycemic agents. Annual Review of Medicine. 31, 26172.
- 10.Bastaki S. (2005) Review Diabetes mellitus and its treatment. , Int J Diabetes & Metabolism 13, 111-134.
- 11.Celeste C L, Quianzon M D, Issam E, Cheikh.History of current non-insulin medications for diabetes mellitus. doi: 10.3402/jchimp.v2i3.1908. , Journal of Community Hospital Internal Medicine Perspectives 2, 19081.
- 12.Kleppinger E L, Vivian E M. (2003) Pramlintide for the treatment of diabetes mellitus. Ann Pharmacother. 37, 10829.
- 13.[cited 29 February 2012]Amaryl [Internet].SilverSpring, Maryland: U.S. Food and Drug Administration. Available from:. http://www.accessdata.fda.gov/scripts/cder/drugsatfda/index.cfm?fuseactionSearch. DrugDetails
- 14.Aschcroft F, Proks P. (2002) Sulfonylurea stimulation of insulin secretion. , Diabestes 51, 368-376.
- 15.Aschcroft F, Proks P. (2002) Sulfonylurea stimulation of insulin secretion. , Diabestes 51, 368-376.
- 16.Moller D. (2001) Zhou g., Role of Amp- activated protein kinase in mechanism of metformin action, The journal of clinical investigation. 108, 1167-1174.
- 17.Levine R.Sulfonylureas: background development of the field. Diabetes Care1984;7(supplement1): 3-7.
- 18.Duggleby R, Wang J. (2005) Structure-activity relationships for a new family of sulfonylurea herbicides. , Journal of computer-aided molecular design 9, 801-820.
- 20.Collins R, Peto R.Hennekens ch:blood pressure, stroke, and coronary heart disease. Part 2: short-term reductions in blood pressure: overview of randomized drug trials in their epidemiological context.Lancet,1990,335,p:. 827-838.
- 21.Ghiadoni l, Salvetti A. (2006) Thiazide diuretics in the treatment of hypertension: an update. , Journal of American society of nephrology 17, 26-29.
- 22.Housten M C.THIAZIDE and thiazide-like diuretics in hypertension. , Ann Intern Med; Aug; 103(2), 303.
- 23.Janssens J, Peters T. (1998) Actions of 5- hydroxytriptamine 1 receptor agonist sumatriptan on intergigestive gastrointestinal motility in man. 42, 36-41.
- 24.Bombardier C, Laine I. (2000) Comparison of upper gastrointestinal toxicity of rofecoxib and naproxen in patients with rheumatoid arthritis. , N. ENGL. J. MED 1520-1528.
- 26.Schooley R, Myers R. (2001) A dose- ranging study to evaluate theantiretroviral activity and safety of amprenavir alone and in combination with abacavir in HIV-infected adults with limited antiretroviral experience, Antiviral therapy. 6, 89-96.
- 27.Wire M, khaled M. (2009) Fasoamprenavir\ ritonavir in advanced HIV disease (TRIAD):a randomized study of high-dose, dual-boosted or standard dose fosamprenavir\ ritronavir in HIV-1-infected patients with antiretroviral resistance. , Journal of antimicrobial chemotherapy 64, 398-410.
- 28.Chaverri J, Garcia M. (2012) Antioxidant activity of topiramate: an antiepileptic agent, neurological sciences.
- 29.Woolsey R, Wood A. (1990) A mechanism of d- (+)- sotalol effects on heart rate not related to beta- adreniceptor antagonism, British journal of clinical pharmacology. 30, 195-202.

