Abstract
Background
Immunoglobulins are bio-receptors found embedded in the cell membrane with a biological role that detects the harmful molecules of a test compound. These bio-receptors interface between a biological system and its external environment that transduce information to the effector via intermediate messengers in which its response efficiency usually exhausts at high doses of exposure to external stimuli. The purpose of this review article is, therefore, to elaborate on the computational method for systemic biology which was designed to convert qualitative pharmacological data into the quantitative one that might help to determine the toxicity of a test compound.
Methods
First, acute toxicity studies using different levels of doses prepared from each test compound have been conducted on Balb c mice. Then, blood specimens from the tail and facial veins of each sampled Balb c mouse were collected 3 days before dosing as a reference test and 4 hr after dosing for comparison. The changes in the efficiency of immunoglobulins immune response (ΔIg) after dosing were determined using quantitative immunoassay and the body’s response against the dose as the toxic reaction rate (r) and the toxic severity (s) were finally determined using computational methods as r=d/t-ΔIg mg/sec and (s=r/w×100) %/sec respectively, where (w) represents the body weight of a study animal, (t) represents the period of time at which undesirable bio-physiological responses manifested on treated study animals and (ΔIg) represents the changes in the concentration of immunoglobulins in blood serum after dosing.
Results
The results of different studies revealed that the dose has never limited the toxic property of a test compound but the length of time at which the undesirable side effect was manifested on study animals. The period of time at which adverse effects manifested on treated Balb c mice was inversely related to the amount of dose administered in the oral route. The higher the dose of the administered test compound, the shorter the period of time at which the undesirable side effect was manifested on treated Balb c mice. This means that the adverse effect of test compounds was not because of the dose but rather due to its toxic reaction rate which ultimately determined the toxic severity in the natural process of treated Balb c mice. Balb c mice treated with a dose whose toxic reaction rate was ≤ 0 survived from death whereas Balb c mice treated with a dose that had a toxic reaction rate of > 0 died at different lengths of time after dosing depending on the toxic severity of a test compound. It could be a scientific fact to declare that a test compound is safe when the toxic reaction rate (r) and toxic severity (s) of a dose is ≤ 0 and toxic when it is > 0 in the natural processes of a study animal.
Author Contributions
Academic Editor: Fatma Mohammed Mady, Department of Pharmaceutics, MiniaUniversity.
Checked for plagiarism: Yes
Review by: Single-blind
Copyright © 2024 Yilkal Tariku Belay
 This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Competing interests
The authors have declared that no competing interests exist.
Citation:
Introduction
The body recognizes health hazards via the receptors of biological systems known as the sensory and the immune system 1. These bio-receptors that interface between the body system and its external environment have limited response efficiency which usually declines at high doses of exposure to external stimuli. Sensory systems are biological organs that perform taste, smell, vision, hearing, and visceral sensations that could detect health hazards such as poisonous substances 1. They have instinctive recognition of hazardous stimuli and hence react against them with painful physiological responses such as nausea and vomiting to prevent them from accessing the biological systems. It is hard for the harmful compound to reach the body’s systems because of the strong responses of the body’s sense of taste and smell. For instance, if we serve a hungry pussy cat to drink a pesticide which is milky in colour, it could not drink it due to the fact that it would sense an unpleasant smell. Thus, the sensory system provides primary protection to the body against poisonous substances found in the external environment 1. However, the receptors of these sensory organs have limited biological sensitivity depending on the degree of exposure to recognize health hazards to be able to avoid them accessing the biological systems. The biological and physiological responses of the body systems become less efficient when the bio-receptors of these sensory organs have interacted with both high and low doses of external stimuli. For example, the sensitivity of an auditory system would be lost at both high and low sound frequencies 2, and the sensitivity of taste and smell would be also lost at high and low doses of exposure 1. Let us also remember the pathological and physiological conditions of the body against high and low vitamin and mineral supplements. High doses of vitamin A, for instance, may cause birth defects, and bone and skin disorders3, 4. Too much vitamin C or zinc could cause nausea, diarrhea, and stomach cramps. Vitamin deficiency can also cause pathological and physiological symptoms like fatigue, dry skin, dry hair, depression, poor wound healing, and more. These biological responses clearly show the fact that the dose is to manifest that biological sensitivity that maintains the natural processes of the body systems which actually determines a lifespan.
The toxicity of hazardous compounds could also be recognized by the immunoglobulins’ immune response following catabolism or anabolism depending on its chemical properties 2. Although intensive immunological research has already been conducted on the biological importance of immunoglobulins in humans and animals, there is nothing done on its potential application in the discovery and development of safe therapeutic agents. Immunoglobulins are cell-signaling glycoprotein molecules formed by the white blood cells, mainly B lymphocytes, which play a great role in the defence mechanism and activation of the immune system to help protect the body from different pathogenic microbes and poisonous substances 5. They are found embedded in the cell membrane with a biological power that recognizes the harmful molecules of a test compound and activates the cellular immune system to produce antibodies which are also known as immunoglobulins 5. This means that immunoglobulins serve as a biosensor that detects the harmful molecules of a test compound administered to a study animal. They also serve as bio-conductors by which they cause cell signaling and cell activation to enable the cellular component of the immune system to form new immunoglobulin molecules against the antigen 1. Immunoglobulins are also found freely in the plasma but they are not involved in cell signaling and cell activation mechanisms 5. Generally, immunoglobulins are biological molecules with a high level of sensibility and memorability to antigenic insults against which they normally manifest proportional counter-responses. They increase in concentration in the blood serum when the harmful molecules of a test compound are dispersed in the body and interact with a drug target except those chemical compounds that are directly harmful (suppressive) to the metabolic system which is explained in the next paragraphs. Under normal biological circumstances, the amount of immunoglobulins produced by the cellular immune response following exposure to a test compound is proportional to the number of harmful molecules interacting with its receptor type 5, 8. This means that immunoglobulins are bio-receptors that could label a biological reaction by generating signals proportional to the concentration of harmful molecules of a test compound. Thus, with reference to the changes in the efficiency of immunoglobulins’ immune response against the administered dose, it would be quite possible to deal with the diverse side effects of a test compound being manifested on treated study animals using the computational systemic pharmacology which is explained in detail in the discussion section.
The quantitative change of immunoglobulins after exposure to a test compound simply represents the fight response of the immune system in attempting to neutralize the undesirable side effect. Immunoglobulins are also an antagonist to the reaction of noxious compounds by which they could minimize the toxic severity and toxic reaction rate within the biological processes of humans and animals 6, 7. The toxic severity refers to the magnitude of a biological harm or an injury caused by the dose of a test compound administered to a study subject which is described in detail in the discussion section. The toxic reaction rate, on the other hand, refers to the number of harmful molecules of a test compound that interacted with the binding domain of a receptor type and caused undesirable side effects on the study subjects which is also described in detail in the discussion section.
A toxicity study at different stages of preclinical and clinical trials is a regulatory procedure to investigate the undesirable side effects and mechanism of toxicity of test compounds on the natural processes of living things. Depending on the objective of a toxicity trial, an In vivo or In vitro study design would be employed to evaluate the toxicity of active molecules of test compounds that perhaps trigger a biological signal in different biological systems. The biological responses that would manifest as a result of interaction between a drug receptor and active molecules of a test compound could be desirable pharmacologic effects or undesirable side effects or both responses manifested simultaneously depending on the selectivity or specificity of a molecule of a test compound for its receptor type 8, 9. For example, propranolol, a non-selective β-adrenoceptor blocker, has both desirable and undesirable effects on different biological systems 8, 9. It has a desirable therapeutic effect on the cardiovascular system where it acts as a useful anti-hypertensive agent by reducing cardiac output and vascular resistance while it has an undesirable side effect on the respiratory system where it prevents β2-receptor-induced bronchodilation which may precipitate bronchoconstriction in susceptible individuals 8, 9. On the other hand, verapamil, a calcium channel blocker, is relatively selective by acting as a useful anti-hypertensive agent without causing bronchoconstriction or preventing broncho dilation which is potentially harmful to asthmatic patients 8, 9. These show that other test compounds are more likely to have many targets that could lead to the manifestation of undesirable side effects on some biological systems while it act as a useful therapeutic agent on other biological systems depending on the selectivity or specificity of the molecules of a compound for a receptor type which makes drug discovery and development very challenging.
The response efficiency of the body (the net outcome of the body’s biological reaction against the side effect) would determine the potency of a test compound to manifest undesirable pharmacologic effects. In other words, the amount of a drug required to cause biological harm or injury depends on the ability of the body’s biological reaction in which the immune response plays a great pharmacological role by neutralizing and harmonizing xenobiotics with the biological molecules 1. The differences between the amount of dose required to manifest biological harm or injury reflect how efficient the biological response of the body is against its side effects. For example, the dose of a test compound at 100 mg/kg body weight could cause death to some of the study animals while it is still non-lethal to some other study animals depending on the response efficiency of the body. The response efficiency of the body is primarily dependent on the viability of the different biological systems 10, 11. The function of each biological system is interdependent in which the proper function of one is dependent on the other. This means that the deterioration of one biological system as a result of biological harm being caused by a test compound would lead to the deterioration of other body systems. For example, a test compound which causes dysfunction of the metabolic system would definitely suppress the magnitude of the immune response. Indeed, the undesirable side effects of a test compound manifested in one of the biological systems would be reflected in the immune system as it is the security of the body.
The immune system has a well-orchestrated network of communication with each biological system of the body which allows it to detect the undesirable biological signals being manifested through signaling and activation mechanisms of immunoglobulins. 12, 13. It is the control center of the body’s security through its complex communication network to all body systems by which it detects the harmful molecules of a test compound or antigen and activates the cellular component of the immune system to participate in the immune response against it. This complex communication network helps to localize the diverse side effects of a test compound being manifested in different organ systems into the immune system which perhaps makes toxicity studies relatively easy to monitor.
A lot of experimental studies are, however, conducted in the discovery and development of safe therapeutic agents every year having no consideration of the immunoglobulin immune response which plays a great biological role in the well-being of a study animal. A lot of time and resources are wasted in multi-phase preclinical trials with the hypothetical concept in which the death of a study animal is the final point for a conclusion. The rate of unsuccessful clinical trials is still frustrating where a lot of money is being spent in the development of a therapeutic agent that does not work. During the pre-marketing stage, for instance, the number of unsuccessful clinical trials is greater than the successful ones because of safety issues 14. Even if a drug reaches the market, post-marketing surveillance could still characterize critical adverse effects of a drug that was not observed during preclinical and clinical trials 14. That is the reason why some therapeutic agents are removed from the market because of their public health issues such as mutagenicity, teratogenicity, carcinogenicity and systemic toxicity. The objective of this review article is, therefore, to elaborate the potential use of immunoglobulins immune response in the discovery and development of safe therapeutic agents which is described in detail in the result and discussion sections.
Methods
Search for relevant information
A search for relevant information was made by the author from his published articles and other physiological literature. Original and review articles have been selected, downloaded, and documented on a computer.
Information extraction method
Information has been collected from different articles published in different peer-reviewed journals on acute toxicology. Other immunological studies that have also demonstrated the immunoglobulins’ immune response against antigens were also included in this review article. .
Data processing and reporting
All information collected from different articles has been systematically arranged and processed using a computer package (Adobe Reader Ⅺ, Microsoft Office and Excel 2013). The processed data has been reported thematically under different sections and subsections described under the result and discussion sections.
Results
The results of 6 articles that have been published in different peer-review journals have been included in this section. It is presented under two subsections described as follows:
Acute toxicology of test compounds
The acute toxicity of ethanol and ether test extracts from the dried seed of Aristolochia elegans Mast at different levels of doses administered to 30 Balb c mice orally was evaluated for 10 days 15. There were no signs and symptoms of adverse effects manifested in the first batch of eight mice treated with a single dose of 500 and 1000mg/kg for the first 3- 4 days 15. The appetite of these mice was, however, gradually suppressed and they eventually started dying 7 days after dosing orally 15. All sampled Balb c mice died without having any forceful response against the death on the 7th, 8th and 9th days after dosing orally 15. The second batch of eight mice which were treated with a single dose of 2000 and 3000 mg/kg body weight of both test extracts started dying on the 5th day after dosing in the same route (15). All treated mice died on the 6th and 7th days after exposure to test extracts 15. The first two mice from the third batch of eight Balb c mice, which were treated with 4000 and 5000 mg/kg body weight of both test extracts died on the 4th day and the remaining six mice died on the 5th and 6th days after dosing orally 15. However, there was no sampled mice died within 72 hours after dosing in the same route.
The period of time at which adverse effects manifested on treated mice was dose-dependant 6, 15. The lethal effect of test extracts has been manifested within a short period when a higher dose was administered in the oral route 6, 15. It also remained after a long period of time when a lower dose was administered in the same route. As a result, it has been difficult to determine the minimum lethal dose (LD50) and the maximum nonlethal dose (ED50) of test extracts 6, 15.
Experimental studies have also been conducted on doses of test chemicals prepared from dichlorvos, chlorpyrifos and cypermethrin pesticides at three different levels of doses (10, 50 and 90) mg/kg which were administered to Balb c mice orally and monitored for 5 days 6. These studies have shown that the dose had never limited the toxic property of test chemicals but the rate and severity of undesirable biological responses that has eventually determined the period at which a recognizable adverse effect was manifested on treated mice. The length of time at which adverse effects manifested on treated Balb c mice was inversely related to the amount of a dose administered in the oral route 6. The higher the dose of the administered test chemical, the shorter the period at which the undesirable side effect was manifested on treated Balb c mice 6. This means that the adverse effect of a test chemical was not because of the dose but rather due to the rate of toxic biological reaction which ultimately determined the toxic severity of test chemicals in the natural process of treated Balb c mice 6. The toxic reaction rate (r) and toxic severity (s) of test chemicals were computed using formulas (r=d/t- ΔIg)%/ sec and ( s=r/w×100)%/sec respectively which is explained in detail in the discussion section.
The toxic severity and toxic reaction rate of cypermethrin were higher than the toxic severity and toxic reaction rate of dichlorvos and chlorpyrifos in the three levels of doses prepared from each test chemical (Figure 1 and Figure 2) 6. The toxic severity of the two different levels of doses prepared from dichlorvos was less than the toxic severity of chlorpyrifos (Figure 1) 6. However, the toxic severity of each test chemical was not linearly projected as the dose administered to lab Balb c mice uniformly increased due to differences in the immune response 6. The toxic severity at a dose of 50 and 90 mg/kg prepared from cypermethrin, for instance, had a dis-proportional difference which was administered to different Balb c mice that had different response efficiency of the immune system 6. The toxic reaction rate at a dose of 10 and 50 mg/kg prepared from dichlorvos and chlorpyrifos was slightly different which was not proportional to the amount of dose administered to Balb c mice (Figure 2). The study revealed that the higher the efficiency of the immune response, the lower the toxic severity of test chemicals within the natural process of treated Balb c mice 6. The efficiency of the immune response of Balb c mice treated with a dose at 10 mg/kg of dichlorvos was higher than the Balb c mice treated with a dose at 50 mg/kg of the same test chemical which caused low toxic severity to treated Balb c mice (Figure 1) 6. This means that the toxic severity of a test chemical was affected not only by the dose and its toxic reaction rate but also by the efficiency of the immune response of Balb c mice
Figure 1.Toxic severity (s) of test chemicals at 4 hr after dosing (Belay Y 2019).
Figure 2.Toxic reaction rate (r) of test chemicals at 4 hr after dosing (Belay Y 2019).
Antigens trigger immunoglobulins immune response
Immunoglobulin G (IgG) and Immunoglobulin M (IgM) quantification tests have been conducted to evaluate the efficiency of immunoglobulin’s immune response against the toxicity of test chemicals administered to Balb c mice. Except for one mouse which died approximately at 1:36 hour after dosing with the highest dose prepared from dichlorvos test chemical, about 1 ml of blood sample was collected from the tail and facial veins of each treated Balb c mouse using micro test tubes before treatment as reference test and at 4 hour after treatment for comparison. The quantitative immunoassay at 4 hour after dosing has shown that the lethal doses prepared from chlorpyrifos and cypermethrin have depleted the concentration of IgM in the blood serum(Figure 3). However, quantitative immunoassay performed at the same time has shown that there was no difference in the concentration of IgG in blood serum before and after dosing which is not presented in this article 6.
Figure 3.Changes in the concentration of IgM in blood serum 4 hour after dosing (Belay Y 2019).
The lower doses prepared from the three test chemicals (dichlorvos, chlorpyrifose and cypermethrin), however, increased the concentration of IgM in blood serum after dosing which has decreased the magnitude of toxic severity and toxic reaction rate of test chemicals below zero (Figure 1, Figure 2and Figure 3). The negative result of toxic severity and toxic reaction rate in (Figure 1 and Figure 2) respectively showed that lower doses have boosted the efficiency of IgM immune response and caused the toxicity of test chemicals to become negligible in the body of treated Balb c mice 6. This doesn’t mean that the test chemicals are safe for Balb c mice. The test chemicals at a low dose might manifest recognizable adverse effects when the immune system exhausts its response efficiency against the undesirable side effect in the long run. It is, therefore, necessary to evaluate the response efficiency of the immune system for the adequate period following exposure to a test chemical. This means that the toxic effect of lower doses starts at the biochemical level of an organism which perhaps causes a toxic effect at the cellular level which eventually leads to biological response at the organismal level as the reactive dose increases and the efficiency of the immune response declines 7, 16.
The immunoglobulin immune response inhibits the undesirable side effects of noxious chemicals by agglutination, precipitation, neutralization, or lysis 1. The study by R C Dodel et al in 2004 showed that human immunoglobulin preparation (IVIgG) specifically recognized and inhibited the neurotoxicity of β-amyloid 17. The concentration of total β-amyloid in the cerebrospinal fluid (CSF) has decreased in all Alzheimer’s disease patients following 6 months of treatment with human IVIgG preparations 17. These antibodies selectively target β-amyloid and are capable of antagonizing the potential neurotoxic effects 17. Immunofluorescence studies were performed by Rosalla Elchuk et al in 1970 on blood serum obtained from acute and chronic cases of American trypanosomiasis (Chagas disease). The study has shown that the concentration of IgM was high in the acute stage whereas the concentration of IgG and IgA was high in the chronic stage of the infection 18. This implies the fact that immunoglobulins’ immune response clearly manifests when there is an injurious agent invaded the biological process of an organism and the magnitude of this response is directly proportional to the number of molecules of noxious chemicals. The immunoglobulin immune response is vital for the living organism to maintain homeostasis due to its function to protect organisms from harmful agents 19. It recognizes undesirable biological phenomena caused by a noxious chemical within the biological process of a study animal which helps to determine the safety pharmacology of a test compound.
Discussion
The response efficiency of the body systems determines the toxicity of xenobiotics: Bio-physiological analyses
Toxicity is the ability of xenobiotics to manifest an undesirable biological process on a single cell, a group of cells, a biological system. or the entire body depending on the amount of a molecule of a substance interacting with its receptor type. The previous studies conducted in 2011 and 2019 have shown that the dose has never limited the toxic property of a test compound but the rate and severity of toxic biological responses of treated Balb c mice. The response efficiency of treated Balb c mice has been evaluated with a computational method for systemic biology which has been considered the following research variables: the amount of dose (d) administered to a study animal, The body weight of a study animal (w), the period of time (t) at which undesirable biological responses manifested on treated study animals and the changes in the concentration of immunoglobulins in blood serum before and after dosing. First, acute toxicity study using different levels of doses prepared from different test compounds has been conducted on Balb c mice in the biomedical laboratory at the department of pharmacology and therapeutics, Then, blood specimens from the tail and facial veins of each sampled Balb c mouse have been collected 3 days before dosing as a reference test and at 4 hr after dosing for comparison. The biological responses as toxic reaction rate (r), toxic severity (s), and the changes in the efficiency of immunoglobulins immune response (ΔIg) after dosing was finally determined using mathematical equations as (r=d/t- ΔIg) mg/sec, ( s=r/w×100) %/sec and quantitative immunoassay respectively which are explained in the next paragraphs.
A test compound is made up of molecules of two or more atoms that could bind to a molecular target known as a receptor within the body systems by means of chemical forces that trigger a biological response 20. The magnitude and nature of a biological response were directly proportional to the amount and nature of a test chemical administered to study animals. The immunoglobulin immune response was one of the biological responses that was manifested following the administration of test compounds to study animals. A noxious test chemical elicits undesirable biological phenomena by binding to its drug receptor on the surface or interior of the cell depending on its chemical and physical properties 20. This biological interaction causes cell signaling mechanism that activates the cellular immune component to produce a typical antibody known as immunoglobulins to antagonize the toxic activity of a test compound 20. This means that the number of harmful molecules interacting with drug receptors, which is designated as the toxic reaction rate (r) of a test compound, determines the amount of immunoglobulins to be produced by the immune system. Evaluation of the magnitude of these biological changes per unit of timeduring the period of the experiment helps to determine the toxic and pharmacological properties of a test compound administered to a study animal.
The toxic reaction rate and other undesirable biological responses are directly proportional to the number of harmful molecules of a test compound interacting with its bio-receptor within the biological systems. The previous studies that were conducted in 2019 have shown that the toxic reaction rate increased when the levels of doses administered to study animals also increased. It should be noted that the amount of a dose determines the magnitude of a biological response in which we could be able to recognize its pharmacological effect at the organismal level. Lower doses usually manifest a pharmacological effect at the cellular or molecular level of an organism that we could not be able to recognize during the period of the experiment without the aid of a microscope. We have to kill a laboratory animal to be able to assess the pharmacological effect of a test compound at the cellular level using a microscope. For instance, we would not be able to get a histologic specimen from the kidney, liver, gastrointestinal tract, or lung without killing a study animal. The undesirable pharmacological effect of a test chemical may not be even manifested by the time we kill a study animal for a microscopic examination. Different test chemicals have different periods at which their undesirable biological effect could manifest on treated study animals. The undesirable biological effect of a test compound might be clearly manifested at different periods following exposure depending on the chemical and physical properties as well as the amount of dose of a test chemical and the strength of the immune response of study animals.
The toxic property of a test compound is diverse and has a variety of adverse effects which make experimental pharmacology very challenging. It requires a holistic biological approach in order to be able to analyse it in a harmonized manner. A test compound that is safe for the liver, might be toxic to the kidney, or a test compound that is safe for the respiratory system, might be harmful to the digestive system because of differences in the sensitivity of receptor types or other drug targets found on each organ system. A test compound that is not toxic to a study animal might be toxic to humans because of the differences in the efficiency of the immune response in different animal species. Experimental pharmacology in drug discovery and development has limited scientific value due to the fact that a dose is considered to be the fundamental concept by which the toxicity of a test compound could be avoided which is far from scientific reality. The chemical nature, by which the toxic effect of a test drug is triggered within the biological process, could not be altered by limiting the amount that has to be administered to a study subject. The pharmacological property of a test compound could not be judged by the amount of a dose but rather by the quality of a biological response it has elicited within the body systems.
The pharmacological property of test compounds has been evaluated by assessing the immunoglobulins immune response against its pharmacological effect without killing the study animals for histologic specimens. Immunoglobulins are naturally antagonists to the reaction of noxious compounds and agonists to the reaction of the immune system which helps protect against bodily harm and destruction. First, the immunoglobulins would express themselves with a suppressed or elevated concentration in the blood serum depending on the chemical and physical properties of a test compound administered to a study animal. The period of time at which a significant change in the bio-availability of the different classes of immunoglobulins in blood serum would determine the pharmacological property i.e. the toxic reaction rate and toxic severity which again determine the pathological mechanism of a test chemical within the biological processes. A study conducted by Michael I. Luster and Gary J. Rosenthal in 1993 showed that alterations in cellular and humoral immune responses often accompany asbestosis, fibrosis, malignant mesothelioma, and bronchogenic Carcinoma. Impairments in cell-mediated immunity in humans by asbestos are characterized by decreases in delayed hypersensitivity responses, the numbers of circulating T cells, and T-cell proliferation. In contrast, patients with asbestosis present hyperactive T-cell responses, often manifested by increased levels of serum immunoglobulins and secretory IgA 21.
The toxic reaction rate (r) of a test compound refers to the number of harmful molecules that has penetrated the antagonistic action of immunoglobulins and has manifested undesirable biological response on treated study animals per unit of time. It is directly proportional to the number of harmful molecules of a test drug that has made a bond with the binding domain of a drug receptor and has caused undesirable biological responses in study animals. This means that the number of molecules of a drug that binds with the molecular target known as a drug receptor is proportional to the magnitude of a biological response manifested in study animals following exposure. Immunoglobulin immune response is one of the biological responses that manifest following exposure to a noxious chemical in which its response is also proportional to the number of harmful molecules of a test compound bound with the molecular target within the biological process of study animals (Figure 4). Solid phase anti-mouse sperm antibody binding assay was conducted by Eli D. Schmell et al in 1982 which also showed that immunoglobulins binding was proportional to the amount of antigen. The assay utilized a commercially available bacterial cell wall component called protein A in which the binding of antibody was proportional to its concentration 22.
Figure 4.Mechanism of immunoglobulins formation against harmful antigens (Marshal et al 2018).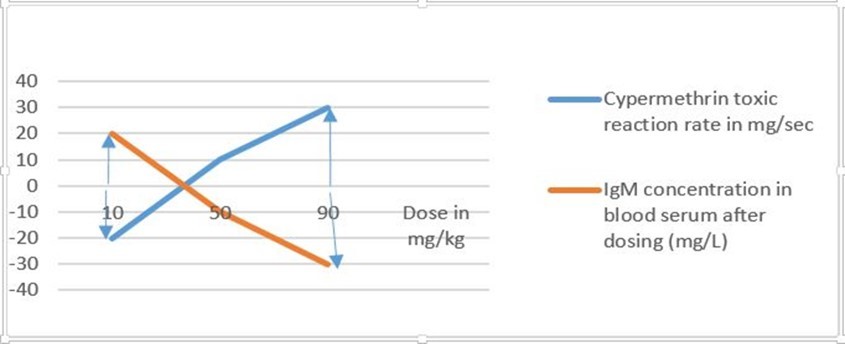
Even though the concentration of immunoglobulins in blood serum following exposure to a test drug is directly proportional to the number of harmful molecules interacting with a bio-receptor, the efficiency of its response was depleted at high doses of exposure to harmful molecules. Previous studies have shown that the higher the toxic reaction rate of a test chemical, the less concentration of immunoglobulins in blood serum and vice versa (Figure 5a & Figure 5b). The study conducted by R.J. Morris and A.F. Williams in 1975 also showed that the number of molecules of antigen bound per molecule of surface immunoglobulins varied from 2 when a large amount of rabbit IgG was on the cell surface, to 4 when a lower amount of IgG densities on the cell surface 23
Figure 5a.The amount of IgM in blood serum at 4 hr after dosing was inversely proportional to the magnitude of toxic reaction rate of doses prepared from cypermethrin test chemical (Belay Y 2019).
Figure 5b.The amount of IgM in blood serum at 4 hr after dosing was inversely proportional to the magnitude of toxic reaction rate of doses prepared from chlorpyrifos test chemical (Belay Y 2019).
The toxic reaction rate of a test compound is directly dose-dependent and inversely time-dependent. It increases as the level of doses increases and the period of time for a biological response decreases. The toxic reaction rate of a test compound is, therefore, equal to the amount of a dose (d) administered to a study animal divided by the period of time (t) at which the adverse effect has manifested on treated study animals minus the antagonistic effect of immunoglobulins within the biological systems. The antagonistic effect of immunoglobulins is proportional to the amount of immunoglobulins produced by the immune system following exposure to a test compound which was designated as (ΔIg). This description could be represented by a mathematical equation as (r=d/t- ΔIg) mg/sec which was described in the previous studies 6, 7. The result of this computational biology helps to know the number of harmful molecules of a test chemical that have interacted with the binding domain of a drug receptor or biological target per unit of time. For example, a test chemical at a dose of 90 mg/kg prepared from chlorpyrifos pesticide has been administered to Balb c mice orally. The amount of IgM in blood serum before dosing was 90 mg/L and at 4 hr after dosing was 80 mg/L. The difference in the amount of IgM in blood serum after dosing) was -10 mg/L. The approximate toxic reaction rate of chlorpyrifos pesticide at a dose of 90 mg/kg within the biological process of Balb c mice, therefore, could be the following:
(r=d/t mg/sec
[ ]
]
r = 10.01mg/sec
This implies the fact that the test compound was not safe for Balb c mice which died at aproximately12: 36 hr after dosing. The toxic reaction rate (r) of chlorpyrifos at 90 mg/kg was 11% higher than the antagonistic effect of the immune response of treated Balb c mice by which the immune response was suppressed (Figure 5a).
The study has shown that the toxic reaction rate and toxic severity of a test compound are inversely proportional to the efficiency of the immune response of study animals. The higher the efficiency of the immune response, the lower the toxic severity and toxic reaction rate of a test chemical would be within the biological processes of a study animal. The toxic reaction rate determines the safety margin of a test compound. A test compound is said to be safe for life when the value of r is ≤ 0. This means that the administered test chemical is harmoniously neutralized with the molecular counterpart of a laboratory animal. The toxic reaction rate also determines the toxic severity of a test chemical which again determines the fate of its pharmacological use in the development of a therapeutic agent. A test chemical is said to be toxic when the value of toxic reaction rate (r) and toxic severity (s) is > 0 (Figure 5a & Figure 5b above).
The toxic severity of a test compound, on the other hand, represents the magnitude of biological harm or injury caused by the amount of administered dose. It is equal to the toxic reaction rate divided by the body weight of a study animal which is expressed in percent per second. This could be again represented by a mathematical equation as ( s=r/w×100) %/sec which was also demonstrated in the previous study 6. The toxic reaction rate of a test chemical at 50 mg/kg prepared from cypermethrin pesticide was 10 mg/Sec. Therefore, the toxic severity of the same dose would be the following:
 ,
, s = 0.32 %/sec
s = 0.32 %/sec
This means that the administered drug at a dose of 50 mg/kg has caused 0.32% biological injury or harm per unit of time. This again implies that the toxic severity of a test chemical is inversely proportional to the body weight of a study animal and directly proportional to the toxic reaction rate as shown in the equation above. This means that the toxic reaction rate (r) per the body weight (w) of the study animal multiplied by the body mass index of an organism would also provide the biological response as toxic severity. This again could be represented by a mathematical equation as follows:
(  ),
),
(Body mass = toxic severity x  ) kg
) kg
(Body mass = ), Body mass =99.14 kg
), Body mass =99.14 kg
This means that the administered drug at a dose of 50 mg/kg has caused 0.32% biological injury or harm per unit of time. This again implies that the toxic severity of a test chemical is inversely proportional to the body weight of a study animal and directly proportional to the toxic reaction rate as shown in the equation above. This means that the toxic reaction rate (r) per the body weight (w) of the study animal multiplied by the body mass index of an organism would also provide the biological response as toxic severity. This again could be represented by a mathematical equation as follows:
This means that the administered test chemical at a dose of 50 mg/kg could manifest a gross biological response on treated study animal with a body mass of approximately 99.14 kg depending on the efficiency of the immune response. The computed toxic severity of a test chemical helps to determine the maximum possible dose which might be safe for life. An integrated biological approach is perhaps the preferable scientific approach in preclinical trials to determine the safety pharmacology of a test compound.
Conclusions
Immunoglobulins serve as a biosensor and bio-conductor by which it recognizes undesirable biological phenomena within the biological structure and causes cell signaling and cell activation. By doing so, it mobilizes the cellular component of the immune system to participate in the immune response. The magnitude and nature of biological response are directly proportional to the amount and nature of a test chemical administered to study animals. The changes in the concentration of immunoglobulins in blood serum after dosing are, however, inversely proportional to the intensity of toxic reaction rate (r) of a test chemical administered to a study animal. The amount of immunoglobulins in blood serum declined as the toxic reaction rate (r) of a test drug becomes high and vice versa.
The toxic reaction rate (r) determined the safety margin of a test chemical. A test chemical is said to be safe for life when the computed value of toxic reaction rate (r) is ≤ 0 for which the difference in the amount of immunoglobulins in blood serum after dosing is > 0.
Acknowledgements
Not applicable
References
- 1.Guyton A, Hall J. (2006) Text book of medical physiology. (Google scholar) , Philadelphia: 465-485.
- 2.Wang Yadong. (2011) Nai Ding, Nayef Ahmar, etal, Sensitivity to temporal modulation rate and spectral bandwidth in the human auditory system: Meg evidence; Articles in. doi: 10.1152/jn.00310.2011 (Google scholar)
- 3.K P west, S K Khatry. (1992) etal; Tolerance of young infants to a single, large doses of Vitamin A: a randomized community trial in Nepal; Bulletin of the world health organization. (Google scholar) 70(6), 733-7339.
- 4.Sidney Q Cohlan. (1954) Congenital Anomalies in the rat produced by excessive intake of vitamin A during pregnancy;. , (PubMed), American Academy of pediatrics 13(6), 556-567.
- 5.Schroeder H, Cavacini L. (2013) Structure and function of the immunoglobulins. 125 (202): S41-S52, doi: 10.1016/j.jaci.2009.09.046. (Google scholar) , J Allergy Clin Immunol
- 6.Y T Belay. (2019) Study of the principles in the first phase of experimental pharmacology: The basic step with assumption hypothesis, BMC Pharmacol. Toxicol. 20, 2-12.
- 7.Morris R, Williams A. (1975) Antigens on mouse and rat lymphocytes recognized by rabbit antiserum against rat brain; the quantitative analysis of a xenogeneic antiserum. (Google Scholar) , Eur. I. Immunol 5, 274-381.
- 8.Illustrated Lippincott. (2015) Reviews, Pharmacology; Drug-Receptor Interactions and Pharmacodynamics; Wolters Kluwer. 6th edition 25-37.
- 9.Basic, Pharmacology Clinical. (2015) Drug Receptors and Pharmacodynamics, Mac Graw-Hill education, a. 13th edition 20-40.
- 10.Vasiliou V, Pappa A, D R Petersen. (2000) Role of aldehyde dehydrogenases in endogenous and xenobiotic metabolism. , Chemico-Biol. Interact 129-1.
- 14.Wu Quire. (2020) Olivier Taboureau, and Karine Audouze; Development of an adverse drug event network to predict drug toxicity. Current Research in Toxicology. 1, 48-55.
- 15.Belay Y. (2011) Study of safety and effectiveness of traditional dosage forms of the seed of Aristolochia elegans Mast against malaria and laboratory investigation of pharmaco-toxicological properties and chemical constituents of its crude extracts, Ann Trop Med Public Health. (Google scholar) 4(1), 33-41.
- 16.Matteis V. (2017) Exposure to inorganic nanoparticles, Routs of entry, Immune response, Biodistribution and in vitro/in vivo toxicity evaluations. , Toxics: 5(29), 10-3390.
- 17.Dodel R, Du Y, Depboylu C, Hampel H, Hoog A et al. (2004) Intravenous immunoglobulins containing antibodies against β-amyloid for the treatment of Alzheimer’s disease. (Google scholar) , J. Neurol Neurosurg Psychiatry 75, 1472-1474.
- 18.Lelchuk R, Dalmasso A, Inglesini C, Alvarez M, Cerisola J. (1970) Immunoglobulin studies in serum of patients with. (Google scholar) , American Trypanosomiasis (Chagas’ disease), Clin. Exp. Immunol 6, 547-555.
- 19.Kazeeva T, Shevelev A. (2007) Unknown functions of immunoglobulins-A. (Google scholar) , Moscow: 72, 603-614.
- 20.Stewart G, Kniazef J, Prezeau L, Rondard P, Pin J. (2012) . Metabotropic Receptors for Glutamate and GABA, InTech: (google scholar) 1.
- 21.Luster M, Rosenthal G. (1993) Chemical agents and the immune response, Environmental perspectives. Research triangle. (Google scholar) 100-219.
