Abstract
In this investigation, Rifabutin dithiocarbamate (RFND) was labeled with Technetium-99m (99mTc) using tricarbonyl technique. The labeled RFND was further characterized in terms of radiochemical purity, stability in saline & serum, in vitro bacterial binding, biodistribution in animal model rats and for scintigraphic accuracy in animal model rabbit. Finally different radiobiological characteristics of the 99mTc(CO)3-RFND was compared with the recently reported 99mTc-RFN. It was observed that the dithiocarbamate form of RFN showed better radiochemical purity, stability in saline, bacterial binding, biodistribution and targeted imaging than the recently reported 99mTc-RFN. These better radiobiological parameters posed 99mTc(CO)3-RFND as a more reliable agent for tuberculosis imaging.
Author Contributions
Academic Editor: Asimul Islam, Centre for Interdisciplinary Research in Basic Sciences
Checked for plagiarism: Yes
Review by: Single-blind
Copyright © 2017 Syed Qaiser Shah, et al
 This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Competing interests
The authors have declared that no competing interests exist.
Citation:
Introduction
In the last century, one of the major achievements of the scientists is the development of vaccines and antibiotics that up to a higher extent eliminated or managed majority of the infectious diseases. Besides such tremendous achievements in the diagnosis and treatment of infectious diseases, infection still remains the focus of investigators and even these days infection is believed to be the major cause of morbidity and mortality1, 2.
Due to the advancement in clinical pathology, the infectious diseases can be detected through simple laboratory tests and successfully treated with appropriate drugs. However, it is observed that a major fraction of those infections resulting in death could be owing to conditions complicated to detect in its early stages. Early and in time detection in such situations could help in appropriate treatment and hence decrease the chances of death3, 4.
Nuclear Medicine Scintigraphic Technology (NMST) provides a different alternative for localization of suspected bacterial infection due its higher sensitivity. In case of deep tissue infection, bone infection, acute life threatening infections which need early appropriate management e.g appendicitis, severe chronic infections occurring due to drug-resistance; and opportunistic infections in immune-compromised persons, one could take advantage of NMST. Such quarries like infection being there or not and its site, severity and potential cause could be answered by using NMST. However, to reply these quarries accurately, the prerequisite is a reliable radio-drug that can accumulate at the site of infection. The radio-drug intended for scintigraphy must answer the above mentioned quarries, but it shall not be toxic, show higher uptake in the target areas, low dose, and low cost easy availability5, 6.
The reported agents7, 11and its derivatives12, 25 have shown promising specific target (infectious area) to non-target (non infectious area) ratios in its very early stages, besides normal circulatory and excretory behavior. However, the appearance of multidrug resistant bacteria’s like Mycobacterium tuberculosis (MBT), is a serious threat for the clinicians to detect and manage such infections in its early time26.
In this scheme, labeling of Rifabutin dithiocarbamate (RFND) (Figure 1.) with 99mTc was examined using tricarbonyl technique. The feasibility of the tricarbonyl labeling procedure is based on the poorly attached H2O of the 99mTc(OH2)3(CO)3]+ precursor which can be easily substituted. The labeled RFND was further characterized in terms of radiochemical purity, stability in saline & serum, in vitro bacterial binding, biodistribution in animal model rats and for scintigraphic accuracy in animal model rabbit. Finally different radiobiological characteristics of the 99mTc(CO)3-RFND was compared with the recently reported 99mTc-RFN27.
Figure 1(b).Rifabutin dithiocarbamate (RFND)
Materials
Rifabutin (RFN) was obtained from Chengdu Yuyang High-Tech Developing Co., Ltd. China, and all chemicals & solvents from Sigma. In this work HPLC of Shimadzu, well counter of Ludlum, Dose calibrator of Capintech and Gamma camera of Nuclearmedizine, were used.
Methods
Derivatization of Rifabutin
Rifabutin (RFN) was derivatized to Rifabutin dithiocarbamate (RFND) using the method reported earlier28. Briefly, 0.002 mol of RFND and 2.4 mg of NaOH were mixed in a clean sterilized vial. Thereafter, 22 ml of tetrahydrofuran (THF) was added to the reaction vial followed by shaking for 30 min in an ice bath. Then, 2 ml carbon disulfide (CS2) was added and left the reaction vial for 8 h in an ice bath for continuous shaking. After that the mixture was processed for continuous stirring up to 12 h at room temperature followed by recovery through re-crystallization. The RFND was characterized by advance spectroscopic techniques.
Synthesis of 99mTc(CO)3-RFND & Radiochemical purity
Sodium pertechnetate 1 mCi (0.2 ml) was mixed with 2 mg (dissolved in 0.4 ml normal saline) of RFND followed by pH adjustment (pH 10) using 0.1 mol / L HCL in a clean nitrogen gas filled sterilized vial. Thereafter, the mixture was transferred to an Isolink kit followed by incubation for optimum labeling at 25 ˚C for 15 min.
High-performance liquid chromatography (HPLC) was used to characterize 99mTc(CO)3-RFND in terms of radiochemical purity by the method reported earlier16 . Briefly, 10 µL of 99mTc(CO)3-RFND was administered to the HPLC system fitted with UV detector operating at 254, and a flow scintillation counter, C-18 column and binary pump. Thereafter, for 15 min, a flow rate of 1 ml / min the column was eluted with water and methanol (W:M). The effluent was collected in separate vials followed by counting for activity.
Mycobacterium Tuberculosis (MBT) Uptake
MBT uptake of 99mTc(CO)3-RFND was assessed adopting the method reported earlier28 . Briefly, 0.8 mL acaetic acid (0.01 M) containing approximately 1 x 108 colony forming units (CFU) of MBT and 0.2 mL sodium phosphate buffer was incubated at 4 °C for 60 min. The mixture was centrifuged at 1500 rpm for 15 min and after removing the supernatant was re-centrifuged after suspending in 1.5 mL sodium phosphate buffer. Subsequently, the supernatant was removed again and the bacterial pellets were counted for activity.
Biodistribution
The percent in vivo uptake of the 99mTc(CO)3-RFND was assessed in healthy and artificially infected animal model rats. The animal was divided into two groups i.e. A and B. To group A animals, approximately 1 x 108Colony Forming Units (CFU) in 0.2 mL saline MBT was intramuscularly injected into the left leg of the anaesthetized rat Sprague-Dawley rat (weight range, 200–250g) for creating infection. After eight hours, equimolar amount of sterile oil was injected to the right leg of the same rat for creating inflammation followed by intravenous admission of 0.5 mCi 99mTc(CO)3-RFND. To group B animals, the above process was repeated without administration of MBT. Thereafter, the rats were sacrificed at different intervals after intravenous injection of radio-drug as per procedure of the Pakistan Nuclear Regulatory Authority (PNRA), Ethics Committee, Pakistan Atomic Energy Commission (PAEC). Thereafter, % in vivo uptake of the 99mTc(CO)3-RFND in one gram of blood, spleen, stomach, intestine, kidney, infected muscle, inflamed and normal muscle was measured using gamma counter.
Imaging with 99mTc(CO)3-RFND
Healthy rabbits (weight: 3.0 to 4.0 kg) were used in the assessment of imaging profile of the instant radio-drug. 0.5 mL MBT containing 1 x 108CFU was injected to the left leg of the healthy rabbit and after 08 h, to the right leg of the same rabbit 0.5 mL sterile oil was injected. Finally, the rabbit was placed face up on the bed of the gamma camera followed by intravenous injection of 2 mCi 99mTc(CO)3-RFND. Whole body images were acquired using Low Energy General Purpose (LEGP) collimator at different intervals.
Statistical Analysis
Results are expressed as % Injected dose / gram or ratios ± SEM and statistical analysis were executed using the student t -test
Results and Discussion
Chemistry of 99mTc(CO)3-RFND
Rifabutin (RFN) (Figure 1 (a)) was derivatized to Rifabutin dithiocarbamate (RFND) Figure 1 (b) followed by labeling with 99mTc using tricarbonyl technique giving the proposed structure Figure 1 (c) with tetrahedral geometry and stiochiometery of ligand:99mTc(CO)3 as 1:2. The two sulfur atoms of the bidentate RFND in the fac-[99mTc(CO)3(H2O)]+ precursor readily displaced H2O to give the required 99mTc(CO)3-RFND complex.
Synthesis of 99mTc(CO)3-RFND & Radiochemical purity
The combined HPLC radiochromatogram of 99mTc(CO)3-RFND and 99mTc-RFN is shown in Figure 2. The blue line described 99mTc-RFN and the red 99mTc(CO)3-RFND. In both lines (blue and red) two markedly different peaks were observed at different retention times. In blue line signal appear at retention 4.1 min represent the free and hydrolyzed technetium-99m and 11.00 min represent the labeled moiety. The red line also showed two different peaks one at 3.3 and the second at 10.2 min. The signal appear at 3.3 min of retention represent the unlabeled while the one at 10.2 min of retention, the labeled dithiocarbamate.
Figure 2.Combined HPLC trace of 99mTc(CO)3- RFND (red trace) and 99mTc-RFN (blue trace)
In normal saline the 99mTc(CO)3-RFND showed normal profile like 99mTc-RFN at room temperature up to 240 min after reconstitution. The combine radiochemical stability of 99mTc(CO)3-RFND and 99mTc-RFN is shown in Figure 3. The blue trace represent the radiochemical stability profile of the 99mTc(CO)3-RFND up to 240 min, wherein it was observed that the 99mTc(CO)3-RFND has shown more than 90 % stability. In normal saline the observed radiochemical purities at 1, 30, 60, 90, 120 and 240 min after reconstitution were 94.70 ± 0.24, 99.25 ± 0.20, 98.00 ± 0.18, 96.40 ± 0.00, 95.00 ± 0.16 and 93.10 ± 0.20 %.
Figure 3.Stability of 99mTc(CO)3-RFND (blue trace ) & 99mTc-RFN (red trace) in normal saline at room temperature
However, the red trace showed radiochemical profile of the 99mTc-RFN in normal saline up to 240 min after reconstitution portray almost similar trailing patron. The radiochemical purities calculated at 1, 30, 60, 90, 120 and 240 min after reconstitution were 92.50 ± 0.16 %, 98.45 ± 0.18 %, 97.30 ± 0.20 %, 95.10 ± 0.16 %, 94.20 ± 0.14 % and 90.70 ± 0.70 % respectively27. The 99mTc-RFN freshly prepared showed more than 90 % radiochemical purity up to 240 min.
Behavior of 99mTc(CO)3-RFND in Human Serum
The combined behavior of the 99mTc(CO)3-RFND and 99mTc-RFN is shown in Figure 4. The blue trace was represented the behavior of the 99mTc(CO)3-RFND in human serum at 37 °C up to 16 hrs after reconstitution. The profile of 99mTc(CO)3-RFND at 0, 2, 4, 6, 8, 10, 12, 14 and 16 hrs after reconstitution were 99.00 ± 0.50, 96.15 ±0.44, 93.20 ± 0.42, 91.70 ± 0.44, 90.00 ± 0.46, 88.35 ± 0.40, 87.15 ± 0.46, 86.50 ± 0.44 and 85.80 ± 0.48 % (with overall decay of 13.20 ± 0.15 %) respectively
Figure 4.Stability of 99mTc(CO)3-RFND (Blue Trace ) & 99mTc-RFN (red trace)
However, the red trace was represented the behavior of the 99mTc-RFN in human serum at 37 °C up to 16 hrs after reconstitution. The profile of 99mTc-RFN at at 0, 2, 4, 6, 8, 10, 12, 14 and 16 hrs after reconstitution were 98.15 ± 0.22 %, 93.30 ± 0.18 %, 90.75 ± 0.24 %, 98.10 ± 0.26 %, 88.35 ± 0.18 %, 86.70 ± 0.20 %, 84.10 ± 0.22 %, and 82.95 ± 0.16 % (with overall decay of 15.20 ± 0.45 %) respectively. Both the radiolabeled had shown permissible less than unwanted species than the limit of decay set US and British pharmacopeia.
Mycobacterium Tuberculosis (MBT) Uptake
The combine MBT uptake of 99mTc(CO)3-RFND and 99mTc labeled is shown in Figure 5. The MBT uptake of 99mTc(CO)3-RFND observed in case of live MBT at 30, 60, 90 and 120 min were 34.50 ± 1.5, 52.50 ± 1.3, 72.25 ± 1.00, and 60.75 ± 1.8 % respectively. In case of heat killed MBT the uptake patron of 99mTc(CO)3-RFND showed analogous behavior to the heat killed up take of 99mTc-RFN. The MBT uptake of 99mTc(CO)3-RFND in case of heat killed MBT recorded at 30, 60, 90 and 120 min were 15.00 ± 1.2, 17.50 ± 1.4, 17.50 ± 0.8 and 15.00 ± 0.9 % respectively. In case of 99mTc-RFN uptake in live strain of MBT at 30, 60, 90 and 120 min were 22.50 ± 0.8, 46.50 ± 1.4, 62.00 ± 1.2 and 55.75 ± 1.10 % respectively. However, in case of heat killed MBT the uptake patron 30, 60, 90 and 120 min showed close correlation with uptake of 99mTc(CO)3-RFND. The uptake observed in case of heat killed MBT were 12.50 ± 1.5, 15.00 ± 1.2, 17.50 ± 1.00 and 15.00 ± 1.4 respectively. The combine uptake profile of 99mTc(CO)3-RFND and 99mTc-RFN in MBT (both live and heat killed) demonstrated almost similar uptake behavior27.
Figure 5.Bacterial binding behavior of 99mTc(CO)3-RFND & 99mTc-RFN
Biodistribution in Animal Model Rats
The percent distribution of 99mTc(CO)3-RFND in one gram of blood, liver, spleen, stomach, intestine, kidney, infected muscle, inflamed and normal muscle of animal model rats is summarized in Table 1. In blood of animal model rats infected with live strain of MBT the activity distribution of 99mTc(CO)3-RFND observed at 30, 60, 90 and 120 min was 21.75 ± 0.24, 10.35 ± 0.22, 9.00 ± 0.28 and 4.35 ± 0.20 %, while in heat killed MBT injected model the distribution observed at 30, 60, 90 and 120 min was 21.25 ± 0.18, 10.75 ± 0.16, 9.20 ± 0.10 and 4.50 ± 0.20 % respectively. In comparison the distribution of 99mTc-RFN showed almost similar patron, initially a high distribution was noticed which gradually went down from 18.95 ± 0.46, 12.50 ± 0.40, 10.50 ± 0.44 and 6.00 ± 0.34 % respectively. In case of heat killed MBT the distribution of 99mTc-RFN activity in animal model showed similar behavior as notice in case of 99mTc(CO)3-RFND27. No significant change in distribution of activity in different animal models was seen.
Table 1. The percent distribution of 99mTc(CO)3-RFND in animal model rats| Organs /tissues (gm) | Absorption of 99mTc (CO)3 -RFND per gram of different organs at different times | |||||||
| In live Mycobacterium tuberculosis | In heat killed Mycobacterium tuberculosis | |||||||
| Time (min) | 30 | 60 | 90 | 120 | 30 | 60 | 90 | 120 |
| Infected muscle | 6.90 ± 0.24 | 10.50 ± 0.20 | 15.60 ± 0.18 | 13.00 ± 0.22 | 2.00 ± 0.26 | 2.50 ± 0.22 | 2.50 ± 0.22 | 2.00 ± 0.24 |
| Inflamed muscle | 3.00 ± 0.10 | 3.50 ± 0.15 | 3.00 ± 0.20 | 3.00 ± 0.15 | 3.00 ± 0.10 | 3.50 ± 0.15 | 3.00 ± 0.20 | 2.50 ± 0.15 |
| Normal muscle | 2.50 ± 0.15 | 3.00 ± 0.20 | 2.50 ± 0.20 | 2.50 ± 0.10 | 2.50 ± 0.10 | 3.00 ± 0.16 | 2.50 ± 0.10 | 2.50 ± 0.18 |
| Blood | 21.75 ± 0.24 | 10.35 ± 0.22 | 9.00 ± 0.28 | 4.35 ± 0.20 | 21.25 ± 0.18 | 10.75 ± 0.16 | 9.20 ± 0.10 | 4.50 ± 0.20 |
| Liver | 20.90 ± 0.16 | 11.00 ± 0.20 | 9.50 ± 0.18 | 5.80 ± 0.14 | 20.50 ± 0.26 | 11.45 ± 0.28 | 9.85 ± 0.22 | 6.00 ± 0.24 |
| Spleen | 10.00 ± 0.28 | 9.25 ± 0.24 | 6.15 ± 0.20 | 4.25 ± 0.28 | 10.45 ± 0.18 | 9.55 ± 0.20 | 6.30 ± 0.16 | 4.30 ± 0.18 |
| Kidney | 9.15 ± 0.24 | 18.20 ± 0.20 | 19.10 ± 0.26 | 22.90 ± 0.28 | 9.30 ± 0.22 | 17.65 ± 0.30 | 20.10 ± 0.24 | 22.35 ±0.26 |
| Stomach & intestines | 9.75 ± 0.34 | 8.00 ± 0.32 | 7.10 ± 0.28 | 4.50 ± 0.30 | 9.55 ± 0.28 | 7.85 ± 0.24 | 6.95 ± 0.22 | 4.55 ± 0.20 |
The activity distribution of 99mTc(CO)3-RFND in other organs of the animal models have shown almost analogous patron as observed in case of 99mTc-RFN. It was observed that the concentration of the 99mTc(CO)3-RFND in other organs like liver, spleen, stomach and intestine considerably went down up to 120 min after injection. The concentration of 99mTc(CO)3-RFND in liver of animal model infected with live strain of MBT at 30, 60, 90 and 120 min was 20.90 ± 0.16, 11.00 ± 0.2, 09.50 ± 0.18 and 5.80 ± 0.14 % and in animal model infected with heat killed MBT was 20.50 ± 0.26, 11.45 ± 0.28, 9.85 ± 0.22 and 6.00 ± 0.24 % respectively. The distribution of 99mTc(CO)3-RFND in liver (one gram) of animal models have shown similar behavior with no significant difference with the reported radio-labeleds. The quantity of 99mTc-RFN observed in liver of animal models infected with live strain at different intervals were 18.95 ± 0.46 %, 12.50 ± 0.40 %, 10.50 ± 0.44 % and 6.00 ± 0.34 %, while in case of animal model infected with heat killed strain of MBT 18.50 ± 0.38 %, 12.40 ± 0.38 %, 10.30 ± 0.40 %, and 6.10 ± 0.30 % respectively27.
A similar distribution patron has been observed in spleen of animal models. The activity distribution of 99mTc(CO)3-RFND observed in spleen of animal model infected with live strain of MBT at 30, 60, 90, and 120 min was 10.00 ± 0.28, 9.25 ± 0.24, 6.15 ± 0.20 and 4.25 ± 0.28 % while in model infected with heat killed MBT was 10.45 ± 0.18, 9.55 ± 0.20, 6.30 ± 0.16 and 4.30 ± 0.18 % respectively. The distribution of 99mTc(CO)3-RFND activity in animal models have shown similar patron what we had observed in case of 99mTc-RFN. The level of activity seen in animal model infected with live strain of MBT in case of 99mTc-RFN at at 30, 60, 90, and 120 min were 10.15 ± 0.38 %, 9.85 ± 0.40 %, 6.90 ± 0.36 % & 4.50 ± 0.38 % and in heat killed MBT model was 9.95 ± 0.55 % 9.35 ± 0.30 %, 6.80 ± 0.50 %, and 4.30 ± 0.44 %
In stomach and intestine a similar distribution of 99mTc(CO)3-RFND was seen, as what we had observed in case of 99mTc-RFN. The activity distribution of 99mTc(CO)3-RFND in animal model infected with live and heat killed MBT at 30, 60, 90, and 120 min were 9.75 ± 0.34, 8.00 ± 0.32, 7.10 ± 0.28 and 4.50 ± 0.30 % and 9.55 ± 0.28, 7.85 ± 0.24, 6.95 ± 0.22 and 4.55 ± 0.20 % respectively. The activity distribution of 99mTc(CO)3-RFND showed close correlation to 99mTc-RFN. In animal model infected with live and heat killed MBT the activity distribution reported at at 30, 60, 90, and 120 min were 9.50 ± 0.30 % 7.80 ± 0.34 % 6.75 ± 0.36 % and 4.30 ± 0.36 % and 8.95 ± 0.30 %, 7.55 ± 0.34 %, 6.45 ± 0.38 %, and 4.40 ± 0.36, respectively.
The distribution of 99mTc(CO)3-RFND in kidney showed similar patron as what we had observed in reported RFN27. It was observed that the activity level was high initially which later on went down. No considerable alterations were observed of activity distribution in animal models infected with live or heat killed MBT. The quantity of 99mTc(CO)3-RFND activity at 30, 60, 90, and 120 min in animal models infected with live and heat killed MBT were 9.15 ± 0.24, 18.20 ± 0.20, 19.10 ± 0.26, 22.90 ± 0.28 and 9.30 ± 0.22, 17.65 ± 0.30, 20.10 ± 0.24 and 22.35 ± 0.26 % respectively. However, using 99mTc-RFN more or less similar results were reported in animal model rats infected with either live and heat killed pathogen.
In animal model rats infected with live strains of MBT it was observed that the distribution of 99mTc(CO)3-RFND activity in the infected muscle was low in preliminary stages, which went up gradually. The recorded activity distribution at 30, 60, 90, and 120 min were 6.90 ± 0.24, 10.50 ± 0.20, 15.60 ± 0.18 and 13.00 ± 0.22 % respectively. However, in animal model infected with heat killed MBT the level at 30, 60, 90, and 120 min were 2.00 ± 0.26, 2.50 ± 0.22, 2.50 ± 0.22 and 2.00 ± 0.24 % respectively. Further, the reported distribution of 99mTc-RFN in animal model infected with live and heat killed pathogen at 30, 60, 90, and 120 min were 5.85 ± 0.44 %, 09.15 ± 0.30 %, 14.15 ± 0.00, 12.85 ± 0.40 and 2.50 ± 0.45 % 3.00 ± 0.50 %, 3.50 ± 0.45 %, and 3.00 ± 0.00 respectively27.
Similarly inconsequential deviations were observed in the distribution of 99mTc(CO)3-RFND activity in the other muscles of the animal models (inflamed and normal). Further, higher distribution of 99mTc(CO)3-RFND in the inflamed muscle was observed in contrast to the normal. However, the level of 99mTc(CO)3-RFND in both muscles went down gradually. The level of 99mTc(CO)3-RFND distribution observed at 30, 60, 90, and 120 min in the inflamed muscle of the animal model infected with live and heat killed MBT were 3.00 ± 0.10, 3.50 ± 0.15, 3.00 ± 0.20, 3.00 ± 0.15 and 3.00 ± 0.10, 3.50 ± 0.15, 3.00 ± 0.20 and 2.50 ± 0.15 % respectively. In case of 99mTc-RFN, the distribution of activity showed almost similar patron. The level of 99mTc-RFN activity reported in the inflamed muscles of the animal models infected with live and heat killed pathogens were 3.50 ± 0.40 %, 3.50 ± 0.45 %, 3.50 ± 0.45 %, 3.00 ± 0.50 and 4.00 ± 0.36 %, 3.50 ± 0.30 %, 3.50 ± 0.00 and 3.00 ± 0.34 % respectively.
`In normal muscles of the animal model rats infected with live and or heat killed pathogen a normal and similar distribution was seen in both cases. The level of 99mTc(CO)3-RFND activity distribution in animal models infected with live and heat killed pathogen at 30, 60, 90, and 120 min were 2.50 ± 0.153.00 ± 0.202.50 ± 0.202.50 ± 0.10 and 2.50 ± 0.103.00 ± 0.162.50 ± 0.10 and 2.50 ± 0.18 % respectively
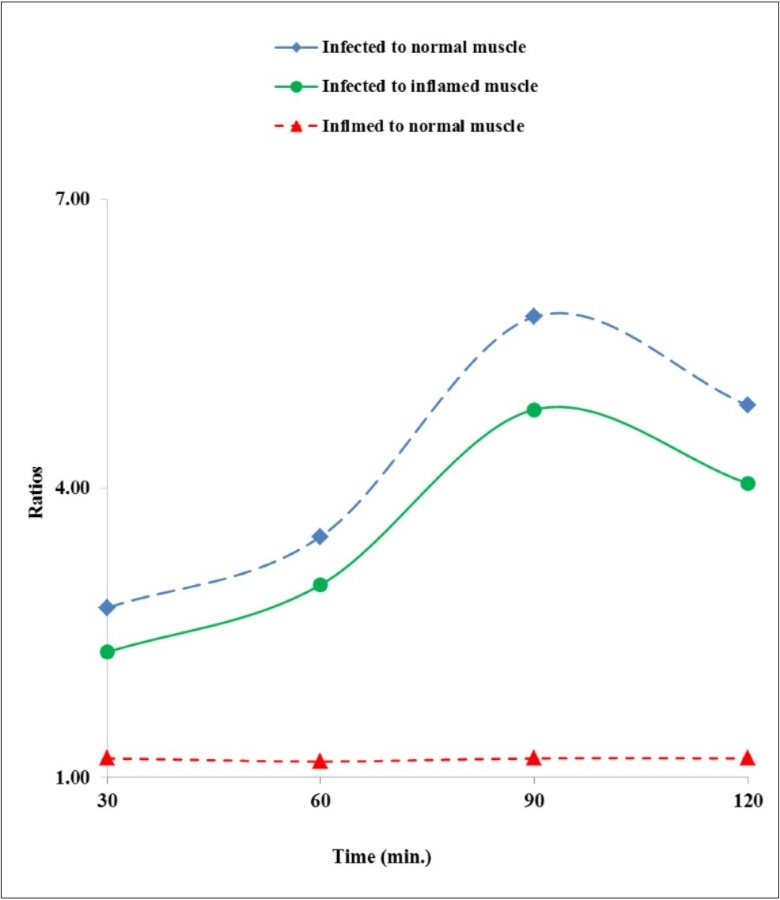
The distribution of activity (ratio-wise) in terms infected to normal, infected to inflamed and inflamed to normal muscles in animal model rats infected are summarized in Figure 6. It was experiential that the concentration of 99mTc(CO)3-RFND in the infected (with live pathogen) muscle was almost six fold advanced than in the normal. Further, the instant profile was not repeated in animal model infected with heat killed pathogen.
Rabbit Scintigraphy
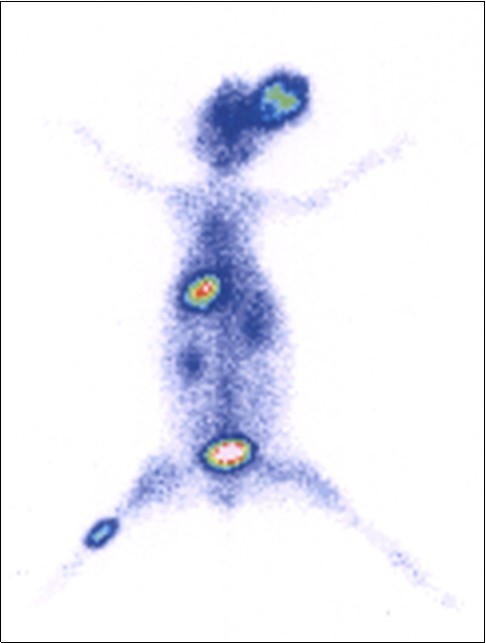
The distribution behavior of 99mTc(CO)3-RFND in animal model infected rabbit with live pathogen is illustrated in Figure 7. After I.V. injection of 99mTc(CO)3-RFND an improved distribution in various organs was observed in comparison with the reported antibiotics. Initially the concentration of 99mTc(CO)3-RFND activity in the infected muscle was lower which went up significantly with time. However, a considerable variation was seen in the distribution of 99mTc(CO)3-RFND activity infected, inflamed and normal muscle even in early phase of the I.V. administration. After 90 min appreciably higher accumulation of 99mTc(CO)3-RFND was observed in the infected muscle and clearly visualized affected area of the rabbit muscle . Further, it was noted that the concentration of 99mTc(CO)3-RFND in blood, liver and spleen was greater in early phases which fade away with and come into view in the infected muscle and kidneys.
Conclusion
In this project Rifabutin (RFN) was derivatized to its dithiocarbamate to enhance binding sites on the RFN so as to enhance its labeling capacity for more realiable radiodiagnostic agent. It was observed that the dithiocarbamate form of RFN showed better radiochemical purity, stability in saline, bacterial binding, biodistribution and targeted imaging than the 99mTc-RFN. These better radiobiological parameters posed 99mTc(CO)3-RFND as a more reliable agent for tuberculosis imaging
References
- 1.Litzler P Y, Manrique A, Etienne M, Salles A, Edet-Sanson A et al. (2010) Leukocyte SPECT/CT for Detecting Infection of Left-Ventricular-Assist Devices: Preliminary Results. , Journal of Nuclear Medicine 51, 1044-1048.
- 2.Nanni C, Errani C, Boriani L, Fantini L, Ambrosini V et al. (2010) 68Ga-Citrate PET/CT for Evaluating Patients with Infections of the Bone: Preliminary Results. , Journal of Nuclear Medicine 51, 1932-1936.
- 3.K E Britton, Vinjamuri S, Hall A V, Solanki K, Q H Siraj et al. (1997) Clinical evaluation of technetium-99m infecton for the localization of bacterial infection. , European Journal of Nuclear Medicine 24, 553-556.
- 4.Mirbolooki M R, Upadhyay S K, Constantinescu C C, Pan M L, Mukherjee J. (2014) Adrenergic pathway activation enhances brown adipose tissue metabolism: A [18 F]FDG PET/CT study in mice. , Nuclear Medicine and Biology 41, 10-16.
- 5.Filippi L, Uccioli L, Giurato L, Schillaci O. (2009) Diabetic foot infection: usefulness of SPECT/CT for 99mTc-HMPAO-labeled leukocyte imaging. , Journal of Nuclear Medicine 50, 1042-1046.
- 6.Wang Y, Chen L, Liu X, Cheng D, Liu G et al. (2013) Detection of Aspergillus fumigatus pulmonary fungal infections in mice with 99mTc-labeledMORF oligomers targeting ribosomal RNA. , Nuclear Medicine and Biology 40, 89-96.
- 7.Belloli S, Brioschi A, L S Politi, Ronchetti F, Calderoni S et al. (2013) Characterization of biological features of a rat F98 GBM model: a PET-MRI study with [18F]FAZA and [18F]FDG. , Nuclear Medicine and Biology 40, 831-840.
- 8.Mirbolooki M R, Constantinescu C C, Pan M L, Mukherjee J. (2011) Quantitative assessment of brown adipose tissue metabolic activity and volume using 18F-FDG PET/CT and β3-adrenergic receptor activation. , EJNMMI Research 30, 1-11.
- 9.Wang Y, Chen L, Liu X, Cheng D, Liu G et al. (2013) Detection of Aspergillus fumigatus pulmonary fungal infections in mice with 99mTc-labeled MORF oligomers targeting ribosomal RNA. , Nuclear Medicine and Biology 40, 89-96.
- 10.Liu X, Cheng D, Gray B D, Wang Y, Akalin A et al. (2012) Radiolabeled Zn-DPA as a potential infection imaging agent. , Nuclear Medicine and Biology 39, 709-714.
- 11.W D Bruggen, C P Bleeker-Rovers, Otto C, O C Boerman, Gotthardt M et al. (2009) . PET and SPECT in Osteomyelitis and Prosthetic Bone and Joint Infections: A Systematic Review. Seminars in Nuclear Medicine 40, 3-15.
- 12.Martins APD, Ossojr J A. (2013) Thermal diffusion of 67Ga from irradiated Zn targets. Applied Radiations and Isotopes. 82, 279-282.
- 13.Liu Z, Wyffels L, Barber C, Hui M, J M Woolfenden. (2011) A (99m)Tc-labeled dual-domain cytokine ligand for imaging of inflammation. , Nuclear Medicine and Biology 38, 795-805.
- 14.Oh S J, Ryu J S, Shin J W, Yoon E J, Ha J et al. (2002) Synthesis of 99mTc-ciprofloxacin by different methods and its biodistribution. Applied Radiations and Isotopes. 57, 193-200.
- 15.Chattopadhyay S, Das M K, Sarkar B R, Ramamoorthy N. (1997) Simple procedure for the preparation of iron-free 67Ga from an irradiated copper target—Use of ascorbic acid. 48, 211-212.
- 16.Ghany E A, Kolaly EMT, Amine A M, Sayed A S, Gelil A F. (2005) Synthesis of 99mTc-pefloxacin: A new targeting agent for infectious foci. , Journal of Radioanalytical Chemistry 266, 131-139.
- 17.Roohi S, Mushtaq A, Jehangir M, Malik S A. (2006) Synthesis, quality control and biodistribution of 99mTc-Kanamycin. , Journal of Radioanalytical and Nuclear Chemistry 267, 561-566.
- 18.Motaleb M A. (2009) Preparation, quality control and stability of 99mTc-sparafloxacin complex, a novel agent for detecting sites of infection. , Journal of labeled Compound and Radiopharmaceuticals 52, 415-418.
- 19.Lambrecht F, Yurt K, Durkan P. (2008) Preparation, quality control and stability of 99mTc-cefuroxime axetil. , Journal of Radioanalytical and Nuclear Chemistry 275, 161-164.
- 20.Zhang J, Guo H, Zhang S, Lin Y, Wang X. (2008) Synthesis and biodistribution of a novel 99mTcN complex of ciprofloxacin dithiocarbamate as a potential agent for infection imaging. , Bioorganic & Medicinal Chemistry Letters 19, 5168-5170.
- 21.Chattopadhyay S, Das S S, Chandra S, De K, Mishra M et al. (2010) Synthesis and evaluation of (99m)Tc-moxifloxacin, a potential infection specific imaging agent. , Applied Radiations and Isotopes 68, 314-316.
- 22.Shah S Q, Khan A U, Khan M R. (2010) Radiosynthesis and biodistribution of (99m)Tc-rifampicin: a novel radiotracer for in-vivo infection imaging. Applied Radiations and Isotopes. 68, 2255-2260.
- 23.Shah S Q, Khan M R. (2011) Radiosynthesis and biological evaluation of the (99m)Tc-tricarbonyl moxifloxacin dithiocarbamate complex as a potential Staphylococcus aureus infection radiotracer. , Applied Radiations and Isotopes 69, 686-690.
- 24.Shah S Q, Khan A U, Khan M R. (2011) Radiosynthesis and biological evolution of 99mTc(CO)3–sitafloxacin dithiocarbamate complex: a promising Staphylococcus aureus infection radiotracer. , Journal of Radioanalytical and Nuclear Chemistry 288-131.
- 25.Oh S J, Ryu J S, Shin J W, Yoon E J, Ha J et al. (2002) Synthesis of 99mTc-ciprofloxacin by different methods and its biodistribution. Applied Radiations and Isotopes. 57, 193-200.
- 26.Kaul A, Hazari P P, Rawat H, Singh B, Kalawat T C et al. (2013) Preliminary evaluation of technetium-99m-labeled ceftriaxone: infection imaging agent for the clinical diagnosis of orthopedic infection. , International Journal of Infectious Diseases 17, 263-270.
Cited by (1)
- 1.Shah S. Q., Ullah N., 2019, Preclinical Evaluation of 99mTc-Ethambutol, an Alternative Tuberculosis Diagnostic Tool, Radiochemistry, 61(2), 233, 10.1134/S1066362219020176