GATES/GEB as the Best Thermodynamic Approach to Electrolytic Redox Systems - a Review
Abstract
The Generalized Approach To Electrolytic Systems (GATES) provides the best possible thermodynamic formulation of redox and non-redox, equilibrium and metastable, mono-, two- and three-phase systems, with all attainable/pre-selected physicochemical knowledge involved, without any simplifying assumptions made for calculation purposes, where different species may occur in batch or dynamic systems, of any degree of complexity. The Generalized Electron Balance (GEB) is the key concept completing the set of algebraic balances referred to redox systems, described according to GATES/GEB ⊂ GATES principles. The GEB, considered as the law of Nature, is fully compatible with charge and concentration balances, and relations for the corresponding equilibrium constants. Within GATES, the electrolytic systems are resolvable with use of MATLAB, or other iterative computer programs, if all necessary physicochemical knowledge is attainable.
Author Contributions
Academic Editor: Mohammad Tavakkoli, Shahid Chamran University of Ahvaz, Ahvaz, Iran.
Checked for plagiarism: Yes
Review by: Single-blind
Copyright © 2020 Anna M. Michałowska-Kaczmarczyk
 This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Competing interests
The authors have declared that no competing interests exist.
Citation:
Introduction
The paper refers to fundamental/general regularities obligatory for electrolytic systems. The linear combination f12 = 2∙f(O) – f(H) of elemental balances: f1 = f(H) for Y1 = H and f2 = f(O) for Y2 = O, is the general, key relation distinguishing between electrolytic redox and non-redox systems in aqueous media 1, 2, 3, 4, 5, 6, 7. The f12 is put in context with charge balance (f0 = ChB) and other, elemental and/or core balances fk = f(Yk) (k=3,…,K; Yk ≠ H, O), related to the system in question. It is stated that f12 is (1o) linearly independent on f0,f3,…,fK for a redox system, or (2o) linearly dependent on f0,f3,…,fK when related to a non-redox system, and thus the independency/dependency property of f12 (3o) provides a rigorous criterion distinguishing between redox and non-redox systems 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18. As the independent balance for a redox system, (4o) f12 is considered as the primary form of Generalized Electron Balance (GEB). f12 = pr-GEB, according to Approach II to GEB. (5o) f12 is fully compatible with f0,f3,…,fK, and (6o) f12 completes the set of K equations f0,f12,f3,…,fK, necessary for thermodynamic solution of a redox system according to Generalized Approach to Redox Systems (GATES) 1, 19; (7o) The set of K–1 balances f0,f3,…,fK is necessary for the solution of a non-redox system; (8o) all relevant, available physicochemical knowledge can be applied for this formulation. Formulation of f12 within GATES/GEB ⊂ GATES (9o) needs none prior knowledge of oxidation numbers (ONs) for all elements in components forming any redox system, and in the species of the system thus formed, where (10o) oxidants and reductants are not indicated a priori20, 21 (11o) Stoichiometry, oxidation numbers, oxidants, reductants and equivalent mass (as GEM 3, 22, 23) are derivative concepts only. As such, (12o) f12 is the hidden connection of physicochemical laws, and (13o) f12 is the breakthrough in thermodynamic theory of electrolytic redox systems. Within GATES, f12 (14o) indicates the unique, distinctive role of the two elements: Y1 = H and Y2 = O in mathematical description of electrolytic (redox and non-redox) systems of any degree of complexity, limited only by the quantitative, physicochemical knowledge related to the system in question, and GATES/GEB provides the best thermodynamic approach to electrolytic (static and dynamic) redox systems, of any degree of complexity. The GEB is perceived as a law of Nature 2, 19, 24, 25, resulting from the charge and elements conservation rules, and GATES/GEB is an example of excellent paradigm 7, 19. All the relations: balances and expressions for equilibrium constants are of algebraic nature, quite different from the nature of stoichiometric reaction notations, that are used in GATES only in context with formulation of the related equilibrium constants according to mass action law. GATES is the best tool applicable for transformation of physicochemical knowledge into algebraic relations, and GEB fills the gap between chemistry and physics of electrolytic redox systems. The f12 formulation within GATES, designed primarily to aqueous media, can be extended on mixed-solvent media, where amphiprotic co-solvent(s) are involved 6, 26, 27.
The GATES formulation is applicable for non-redox and redox systems of any degree of complexity. Applicability of GATES is limited only by the degree of physicochemical knowledge, expressed by a complete set of mutually independent equilibrium constants related to the system in question.
Static (batch) and dynamic electrolytic systems are resolvable within GATES, formulated (1992) by Michałowski 1. The dynamic procedure, most commonly applied in laboratory practice, is the titration, where titrant (T) is added into titrand (D), and the D+T system is thus formed. In D+T systems,considered in chemical analysis and physicochemical studies, different (acid–base, redox, complexation or/and precipitation, extended on two- and three-phase (in liquid-liquid extraction systems 28, 29) types of reactions may occur simultaneously and/or sequentially. Moreover, a particular type of reaction, e.g. complexation, can be exemplified there by different representatives, e.g. different ligands.
In this paper, we concern mainly some electrolytic redox systems, formulated according to GATES/GEB principles, with GEB discovered by Michałowski (1992, 2005) according to the Approaches (I, II) to GEB. The Approach I to GEB 29, 30, 31, 32, 33, 34 is based on the principle of a common pool of electrons introduced by electron-active elements, named, in convention of “card game”, as “players”. The electron-non-active elements are named here as “fans”, and electrons are considered as “money”, transferred between “players”; the “fans” accounts are intact, in this convention 2. The Approach I to GEB, named also as the ‘short’ version of GEB, needs the knowledge of oxidation degrees for all elements in the species participating the system considered. Such a requirement is not obligatory in the Approach II to GEB, based on elemental balances for H and O, outlined in the present paper.
All calculations made within GATES/GEB are related to condensed (liquid, or liquid+solid) phases separated from the environment by diathermal (freely permeable by heat) walls 1. The heat exchange between the system and its environment enables the temperature of the system to be kept constant during the appropriate dynamic process, such as titration, performed in a quasistatic manner. The dynamic buffer capacity formulated for redox systems by Michałowski 35 refers also to titrimetric procedure, like dynamic buffer capacities related to non-redox systems, and formulated also by Michałowski 36, 37, 38, 39, 40, 41.
Complex redox systems, where all types of elementary chemical reactions proceed simultaneously and/or sequentially, were also considered, see 31, 41, 42, 43. In all instances, one can follow measurable quantities (potential E, pH) in dynamic and static processes and gain the information about many details not measurable in real experiments; it particularly refers to dynamic speciation.
Preliminary Notes
Linear Dependency or Independency of Equations
The problem of linear dependency or independency of equations is of fundamental importance in GATES/GEB. Among others, it provides the new criterion distinguishing between redox and non-redox electrolytic systems. An independent equation obtained from charge and elemental and/or core balances related to electrolytic redox systems provides the independent equation known as Generalized Electron Balance (GEB).
Components and Species
The terms: components of an electrolytic system and species in the system are distinguished, for balancing purposes. The components (solvent, solutes) are compounds forming a (static or dynamic) system, and the species Xizi are formed in the system thus obtained. We refer here to aqueous electrolytic systems, where the species Xizi exist as hydrates Xizi.niw, i=1,…, I; zi = 0, ±1, ±2,…is a charge, expressed in elementary charge units, e = F/NA (F – Faraday’s constant, NA – Avogadro’s number), ni = niW = niH2O ≥ 0 is a mean number of water (W=H2O) molecules attached to Xizi ; the case niW=0 is then also admitted. The notation Xizi.niw presents the species as real entities in aqueous media. The niW values are virtually unknown – even n2W for X2z2. n2w = H+1. n2w44, and depend on ionic strength (I) of the solution.
For some reasons, it is justifiable to start the balancing from the numbers of particular entities: N0j – for components (j = 1,…,J) represented by molecules, and Ni – for species (ions and molecules) of i-th kind (i = 1,…,I), where I is the number of kinds of the species.The mono- or two-phase electrolytic system thus obtained involve N1 molecules of H2O and Ni species of i-th kind, Xizi.niw (i=2, 3,…,I), specified briefly as Xizi (Ni, ni), where ni ≡ niW ≡ niH2O. For ordering purposes, we write: H+1 (N2, n2), OH-1 (N3, n3),… , i.e., z2 = 1, z3 = –1
Formation of linear combinations is applicable to check the linear dependency or independency of the balances. A very useful/effective manner for checking/stating the linear dependence of the balances is the transformation of an appropriate system of equations to the identity, 0 = 0 2. For this purpose we try, in all instances, to obtain the simplest form of the linear combination, as indicated in the examples presented below.
A Preliminary Information
Elemental and Core Balances
Within GATES, elemental and/or core balances are distinguished/formulated. The core is a cluster of elements of defined composition (expressed by chemical formula) and external charge; e.g., the core SO4-2 enters the component FeSO4∙xH2O and the species: HSO4-1∙n5H2O, SO4-2∙n6H2O, FeSO4∙n9H2O in aqueous (W=H2O) solutions: D and D+T presented below. Molar concentrations mol/L of the species Xizi.niw is denoted as [Xizi], for brevity. Concentrations of all components and species be expressed in mol/L, and all volumes – in mL.
Static and Dynamic Systems
In further parts of this paper, we consider the D+T system, obtained after addition of V mL of T containing the reagent B (C M) into V0 mL of the solution containing the analyte A (C0 M). The number of mmoles of A equals nA = C0V0 and the number of mmoles of B, added up to a given point of titration, equals nB = CV. The fraction = nB/nA, i.e.,
 …...(1)
…...(1)
is termed as the fraction titrated. The variable introduces a kind of normalization (independence of V0 value) in graphical presentation of the results thus obtained. The will be also very useful in formulation of the equivalence mass according to generalized equivalent mass GEM 1, 2, 3, suggested (1979) by Michałowski 22.
Modelling the electrolytic systems can be realized with use of an iterative computer program, with all attainable physicochemical knowledge involved in the algorithm applied for this purpose. The values of equilibrium constants, interrelating molar concentrations of particular species, are parameters of the corresponding balances.
Formulation of a Redox System According to Approach II to GEB
For comparative purposes, we start from titrand D and titrant T as the static non-redox sub-systems participating the redox D+T system. We consider here:
1) D (V0) composed of FeSO4∙xH2O (N04) + H2SO4 (N05) + H2O (N06) + CO2 (N07);
2) T (V) composed of KMnO4 (N01) + H2O (N02) + CO2 (N03) ;
and
3) D+T (V0+V) as the mixture of D and T, where the following species are formed:
H2O (N1); H+1 (N2, n2), OH-1 (N3, n3), HSO4-1 (N4, n4), SO4-2 (N5, n5), H2CO3 (N6, n6), HCO3-1 (N7, n7),
CO3-2 (N8, n8), Fe+2 (N9, n9), FeOH+1 (N10, n10), FeSO4 (N11, n11), Fe+3 (N12, n12), FeOH+2 (N13, n13),
Fe(OH)2+1 (N14, n14), Fe2(OH)2+4 (N15, n15), FeSO4+1 (N16, n16), Fe(SO4)2-1 (N17, n17), K+1 (N18, n18),
MnO4-1 (N19, n19), MnO4-2 (N20, n20), Mn+3 (N21, n21), MnOH+2 (N22, n22), Mn+2 (N23, n23), MnOH+1 (N24, n24),
MnSO4 (N25, n25) .....(2)
The same notation (N0j, Ni, ni) will be applied, for brevity, also for D and T. The x value in FeSO4∙xH2O 47 is assumed as undefined. The carbonate species in D+T may result from presence of CO2 in water applied as solvent for preparation of D and T.
The complete set of equilibrium constants related to this D+T system is involved in the relations:
[H+1][OH-1] = 10-14.0; [HSO4-1] = 101.8[H+1][SO4-2]; [H2CO3] = 1016.4[H+1]2[CO32]; [HCO3-1] = 1010.1[H+1][CO3-2];
[Fe+3] = [Fe+2]∙10A(E – 0.771); [FeOH+1] =104.5[Fe+2][OH-1]; [FeOH+2] = 1011.0[Fe+3][OH-1];
[Fe(OH)2+1] = 1021.7[Fe+3][OH-1] 2; [Fe2(OH)2+4] = 1021.7[Fe+3] 2[OH-1]2; [FeSO4] = 102.3[Fe+2][SO4-2];
[FeSO4+1] = 104.18[Fe+3][SO4-2]; [Fe(SO4)2-1] = 107.4[Fe+3][SO4-2] 2; [MnO4-1] = [Mn+2]∙105A(E – 1.507) + 8pH;
[MnO4-2] = [Mn+2]∙104A(E – 1.743) + 8pH; [Mn+3] = [Mn+2]∙10A(E – 1.509); [MnOH+2] = 1014.2[Mn+3][OH-1] ...(3)
The notation of balances, similar to one applied e.g. in 7, 18, will be practiced below.
The dynamic system is realized according to titrimetric mode, where V mL of titrant T, added in successive portions into V0 mL of titrand D, and V0+V mL of D+T mixture is obtained at this point of the titration, if the assumption of the volumes additivity is valid; D and T are the sub-systems of the D+T system.
The D Subsystem
We get here the balances:
f0 = ChB
N2 – N3 – N4 – 2N5– N7 – 2N8 + 2N9 + N10 = 0
f1 = f(H)
2N1 + N2(1+2n2) + N3(1+2n3) + N4(1+2n4) + 2N5n5 + N6(2+2n6) + N7(1+2n7) + 2N8n8 +
2N9n9 + N10(1+2n10) + 2N11n11 = 2xN04 + 2N05 + 2N06
f2 = f(O)
N1 + N2n2 + N3(1+n3) + N4(4+n4) + N5(4+n5) + N6(3+n6) + N7(3+n7) + N8(3+n8) +
N9n9 + N10(1+n10) + N11(4+n11) = (4+x)N04 + 4N05 + N06 + 2N07
f12 = 2f(O) – f(H)
-N2 + N3 + 7N4 + 8N5 + 4N6 + 5N7 + 6N8 + N10 + 8N11 = 8N04 + 6N05 + 4N07
–6f3 = –6f(S)
6N04 + 6N05 = 6N4 + 6N5 + 6N11
–2f4 = –2f(Fe)
2N04 = 2N9 + 2N10 + 2N11
–4f5 = –4f(C)
4N07 = 4N6 + 4N7 + 4N8
f12 + f0
6N4 + 6N5 + 4N6 + 4N7 + 4N8 + 2N9 + 2N10 + 8N11 = 8N04 + 6N05 + 4N07 ⇨
6(N4 + 6N5) + 4(N6 + N7 + N8) + 2(N9 + N10) + 8N11 = (2+6)N04 + 6N05 + 4N07
f12 + f0 – 6f3 – 2f4 – 4f5 ⇨ ....(4)
(+1)⋅f(H) + (-2)⋅f(O) + (+6)⋅f(S) + (+2)⋅f(Fe) + (+4)⋅f(C) = ChB (4a)
0 = 0 ....(5)
Note that Fe(+2) species are not oxidized here.
The T S ubsystem
We get here the B alances
f0 = ChB
N2 – N3 – N4 – 2N5– N7 – 2N8 + N18 – N19 = 0
f1 = f(H)
2N1 + N2(1+2n2) + N3(1+2n3) + N6(2+2n6) + N7(1+2n7) + 2N8n8 + 2N19n19 = 2N02
f2 = f(O)
N1 + N2n2 + N3(1+n3) + N6(3+n6) + N7(3+n7) + N8(3+n8) + N19(4+n19) = 4N01 + N02 + 2N03
f12 = 2f2 - f1
-N2 + N3 + 4N6 + 5N7 + 6N8 + 8N19 = 8N01 + 4N03
f12 + f0
4N6 + 4N7 + 4N8 + N18 + 7N19 = 8N01 + 4N03
–7f3 = –7f(Mn)
7N01 = 7N19
–4f4 = –4f(C)
4N03 = 4N6 + 4N7 + 4N8
–f5 = –f(K)
N01 = N18
f12 + f0 – 7f3 – 4f4 – f5 ⇨ ....(6)
(+1)⋅f(H) + (–2)⋅f(O) + (+6)⋅f(S) + (+2)⋅f(Fe) + (+4)⋅f(C) = ChB (6a)
0 = 0.....(5)
Note that MnO4-1 is not reduced here
The D+T System
We get here the balances:
f0 = ChB
N2 – N3 – N4 – 2N5 – N7 – 2N8 + 2N9 + N10 + 3N12 + 2N13 + N14 + 4N15 + N16 – N17 + N18 – N19 - 2N20 + 3N21 + 2N22 + 2N23 + N24 = 0....(7)
f1 = f(H)
2N1 + N2(1+2n2) + N3(1+2n3) + N4(1+2n4) + 2N5n5 + N6(2+2n6) + N7(1+2n7) + 2N8n8 + 2N9n9 + N10(1+2n10) + 2N11n11 + 2N12n12 + N13(1+2n13) + N14(2+2n14) + N15(2+2n15) + 2N16n16 + 2N17n17 + 2N18n18 + 2N19n19 + 2N20n20 + 2N21n21 + N22(1+2n22) + 2N23n23 + N24(1+2n24) + 2N25n25 = 2N02 + 2xN04 + 2N05 + 2N06
f2 = f(O)
N1 + N2n2 + N3(1+n3) + N4(4+n4) + N5(4+n5) + N6(3+n6) + N7(3+n7) + N8(3+n8) + N9n9 + N10(1+n10) + N11(4+n11) + N12n12 + N13(1+n13) + N14(2+n14) + N15(2+n15) + N16(4+n16) + N17(8+n17) + n18n18 + N19(4+n19) + N20(4+n20) +
N21n21 + N22(1+n22) + N23n23 + N24(1+n24) + N25(4+n25) = 4N01 + N02 + 2N03 + (4+x)N04 + 4N05 + N06 + 2N07
f12 = 2f(O) – f(H)
–N2 + N3 + 7N4 + 8N5 + 4N6 + 5N7 + 6N8 + N10 + 8N11 + N13 + 2N14 + 2N15 + 8N16 + 16N17 + 8N19 + 8N20 + N22 + N24 + 8N25 = 8N01 + 4N03 + 8N04 + 6N05 + 4N07 .....(8)
f12 + f0
6N4 + 6N5 + 4N6 + 4N7 + 4N8 + 2N9 + 2N10 + 8N11 + 3N12 + 3N13 + 3N14 + 6N15 + 9N16 + 15N17 + N18 + 7N19
+ 6N20 + 3N21 + 3N22 + 2N23 + 2N24 + 8N25 = 8N01 + 4N03 + 8N04 + 6N05 + 4N07
–6f3 = –6f(S)
6N04 + 6N05 = 6N4 + 6N5 + 6N11 + 6N16 + 12N17 + 6N25 .....(9)
–4f4 = –4f(C)
4N03 + 4N07 = 4N6 + 4N7 + 4N8 .....(10)
–f5 = –f(K)
N01 = N18 .....(11)
Then we have:
A = f12 + f0 – 6f3 – 4f4 – f5 .....(12)
2N9 + 2N10 + 2N11 + 3N12 + 3N13 + 3N14 + 6N15 + 3N16 + 3N17 + 7N19 + 6N20 + 3N21 + 3N22 + 2N23 + 2N24 + 2N25
= 7N01 + 2N04 ⇨
2(N9+N10+N11) + 3(N12+N13+N14 +2N15+N16+N17) + 7N19 + 6N20 + 3(N21+N22) + 2(N23+N24+N25)
= 7N01 + 2N04 .....(13)
f6 = f(Fe)
(N9+N10+N11) + (N12+N13+N14+2N15+N16+N17) = N04 .....(14)
f7 = f(Mn)
N19 + N20 + (N21+N22) + (N23+N24+N25) = N01 .....(15)
The atomic numbers for Fe and Mn are: ZFe = 26 and ZMn = 25, respectively. Then we get
ZFe∙f6 + ZMn∙f7 – (f12 + f0 – 6f3 – 4f4 – f5)
(ZFe–2)(N9+N10+N11) + (ZFe–3)(N12+N13+N14+2N15+N16+N17) + (ZMn–7)N19 + (ZMn–6)N20 +
(ZMn–3)(N21+N22) + (ZMn–2)(N23+N24+N25) = (ZFe–2)N04 + (ZMn–7)N01 ....(16)
Another combination of the equations is also noteworthy. Namely, after addition of the balances
– (f12 + f0 – 6f3 – 4f4 – f5) = –A.....(12a)
7N01 + 2N04 = 2(N9+N10+N11) + 3(N12+N13+N14 +2N15+N16+N17) + 7N19 + 6N20 + 3(N21+N22) + 2(N23+N24+N25)
3f6 = 3f(Fe)
3(N9+N10+N11) + 3(N12+N13+N14+2N15+N16+N17) = 3N04
2f7 = 2f(Mn)
2N19 + 2N20 + 2(N21+N22) + 2(N23+N24+N25) = 2N01
After cancelling similar components in the sum thus obtained and further rearrangements, we get
N9+N10+N11 – (5N19+4N20+N21+N22) = N04 – 5N01 ....(17)
It is the shortest (in terms of the number of its constituents) among the equivalent forms of GEB, obtained from the linear combination
– (f12 + f0 – 6f3 – 4f4 – f5) + 3f6 + 2f7 ⇨ – f0 – (2f2 – f1) + 6f3 + 4f4 + f5 + 3f6 + 2f7 ⇨
– f0 + f1 + (–2)f2 + 6f3 + 4f4 + f5 + 3f6 + 2f7 ⇨ 1⋅f1 + (–2)⋅f2 + 6⋅f3 + 4⋅f4 + 1⋅f5 + 3⋅f6 + 2⋅f7 = f0 ⇨ ....(18)
(+1)⋅f(H) + (–2)⋅f(O) + (+6)⋅f(S) + (+4)⋅f(C) + (+1)⋅f(Na) + (+3)⋅f(Fe) + (+2)⋅f(Mn) = f0 (18a)
The f12 is considered as the primary form of GEB obtained according to Approach II to GEB, f12 = pr-GEB.
Further Remarks
When formulating the balances f1 and f2, it is possible to take into account the formation of water clusters(H2O)𝞴 (N1𝞴, 𝞴 =1, 2,...) in aqueous solutions 49. Writing these balances as follows:
f1 = f(H):
2⋅ + N2 (1 + 2n2) + N3(1 + 2n3) + ...
f2 = f(O) :
 + N2 (1 + n2) + N3(1 + n3) + ...
+ N2 (1 + n2) + N3(1 + n3) + ...
we have:
2f2 – f1 :
– N2 + N3 + ...
i.e., all components related to the clusters are cancelled. The f12 and all linear combinations of f12 with f0 and other balances do not include the terms: N1, niW, x, N02, N06 involved with water.
The coefficients/multipliers at the corresponding balances fk = f(Yk) in Equations 4a, 6a, 9a are equal to oxidation numbers (ON’s) of elements in the corresponding species, see also 20, 21.If oxidation numbers for all elements in the system are known beforehand, the Approach I or II to GEB can be applied optionally, see e.g. 30.
The Balances for the D+T System Expressed in Terms of Concentrations
Applying the Relations:
[Xizi ]∙(V0+V) = 103∙Ni/NA CV = 103∙N01/NA, C0V0 = 103∙N05/NA,
C1V = 103∙N04/NA , C01V0 = 103∙N06/NA , C02V0 = 103∙N08/NA ....(19)
in the balances derived above, we have the optional/equivalent equations for GEB :
[Fe+2] + [FeOH+1] + [FeSO4] – (5[MnO4-1] + 4[MnO4-2] + [Mn+3] + [MnOH+2])
– (C0V0 – 5CV)/(V0+V) = 0 ....(17a)
[H+1] – [OH-1] – [HSO4-1] – 2[SO4-2] – [HCO3-1] – 2[CO3-2] + 2[Fe+2] + [FeOH+1] + 3[Fe+3] + 2[FeOH+2] + [Fe(OH)2+1]+ 4[Fe2(OH)2+4] + [FeSO4+1] – [Fe(SO4)2-1] + [K+1] – [MnO4-1] – 2[MnO4-2] +
3[Mn+3] + 2[MnOH+2] + 2[Mn+2] + [MnOH+1] = 0....(7a)
[HSO4-1] + [SO4-2] + [FeSO4] + [FeSO4+1] + 2[Fe(SO4)2-1] + [MnSO4] – (C0+C01)V0/(V0+V) = 0..(9a)
[H2CO3] + [HCO3-1] + [CO3-2] – (C02V0+C1V)/(V0+V) = 0...(10a)
[K+1] = CV/(V0+V) ....(11a)
[Fe+2] + [FeOH+1] + [FeSO4] + [Fe+3] + [FeOH+2] + [Fe(OH)2+1] + 2[Fe2(OH)2+4] +
[FeSO4+1] + [Fe(SO4)2-1] – C0V0/(V0+V) = 0....(14a)
[MnO4-1] + [MnO4-2] + [Mn+3] + [MnOH+2] + [Mn+2] + [MnOH+1] + [MnSO4] – CV/(V0+V) = 0..(15a)
The simplest form of GEB, expressed by Eq. (17a), is chosen here (arbitrarily) for calculation purposes - for obvious reasons 33. The GEB, formulated in terms of concentrations on the basis of f12 = pr-GEB (Eq. 8) is as follows
– [ H+1] + [OH-1] + 7[HSO4-1] + 8[SO4-2] + 4[H2CO3] + 5[HCO3-1] + 6[CO3-2] + [FeOH+1] +
8[FeSO4] + [FeOH+2] + 2[Fe(OH)2+1] + 2[Fe2(OH)2+4] + 8[FeSO4+1] + 16[Fe(SO4)2-1] +
8[MnO4-1] + 8[MnO4-2] + [MnOH+2] + [MnOH+1] + 8[MnSO4]
– (8CV + 4C1V + 8C0V0 + 6C01V0 + 4C02V0)/(V0+V) = 0 ...(8a)
The GEB, obtained from Eq. 16,
(ZFe–2)([Fe+2]+[FeOH+1]+[FeSO4]) + (ZFe–3)([Fe+3]+[FeOH+2]+[Fe(OH)2+1]+2[Fe2(OH)2+4]+
[FeSO4+1]+[Fe(SO4)2-1]) + (ZMn–7)[MnO4-1] + (ZMn–6)[MnO4-2] + (ZMn–3)([Mn+3]+[MnOH+2]) +
(ZMn–2)([Mn+2]+[MnOH+1]+[MnSO4]) – ((ZFe–2)C0V0 + (ZMn–7)CV)/(V0+V) = 0...(16a)
is identical to that obtained directly from Approach I to GEB, compare with 17, 49. The equivalency of the Approaches (I and II) to GEB is then proved. From Eq. (13) we get
2([Fe+2]+[FeOH+1]+[FeSO4]) + 3([Fe+3]+[FeOH+2]+[Fe(OH)2+1]+2[Fe2(OH)2+4]+[FeSO4+1] +
[Fe(SO4)2-1]) + 7[MnO4-1] + 6[MnO4-2] + 3([Mn+3]+[MnOH+2]) +
2([Mn+2]+[MnOH+1]+[MnSO4]) – (2C0V0 + 7CV)/(V0+V) = 0....(13a)
Eq. 13a is identical with the one obtained from Eq.16a under assumption that ZFe = 0, ZMn = 0 (compare with the ‘debt of honor’ principle/idea suggested in 2 (p. 43).
The set of 6 independent equations: 7a, 9a, 10a, 14a, 15a, 17a is then distinguished. The number of the equations is equal to the number of 6 independent variables, considered as components of the vector:
x = [x1,…,x6]T = (E,pH,pMn2,pFe2,pSO4,pH2CO3)T
where pMn2 = – log[Mn+2], pFe2 = – log[Fe+2], pSO4 = – log[SO4-2], pH2CO3 = – log[H2CO3]. The pXi = - log [Xizi] refer to mutually independent species Xizi.niw. Volume V of the titrant T added is the parameter (not variable) of the system, at defined point of the titration. The relation (11a) is considered as the equality (not equation!); [K+1] can enter immediately Eq. 7a, like a number at defined point of the calculation procedure, defined here by C, V0 and V values.
Calculation Procedure and Graphical Presentation of the Results Obtained
V mL of KMnO4 (C = 0.02 M) is added into V0 = 100 mL of FeSO4 (C0 = 0.01 M) + H2SO4 (C01 = 1.0 M). The simplest form of GEB is there as follows
[Fe+2]+[FeOH+1]+[FeSO4] – (5[MnO4–1]+4[MnO4–2]+[Mn+3]+[MnOH+2]) = (C0V0 – 5CV)/(V0+V)...(23)
The presence of H2SO4 in D affects the related titration curve 1 in Figure 1 at Φ < 0.2; the virtual curve 2 is plotted under assumption that the sulphate complexes: FeSO4, FeSO4+1, Fe(SO4)2-1, MnSO4 were (intentionally) omitted in the related balances, formulated according to GATES/GEB principles. The relations: [FeSO4+1]+[Fe(SO4)2-1] >> [Fe+3] and [FeSO4] > [Fe+2] are valid in the whole Φ-range, and [Mn+3]+[MnOH+2] > [MnO4-1] at Φ > 0.2, see Figure 2.
Figure 1.The E vs. relationships plotted for the D+T System, related to 1 – full model; 2 – with omission of sulphate complexes in the model applied.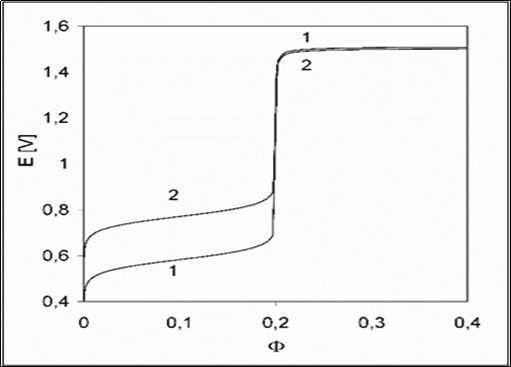
Figure 2.The speciation curves for (A) Fe, (B) Mn species in the D+T system.
All calculations were realized with use of MATLAB iterative computer program 1. Some pairs of (Φe, Ee) points from the close vicinity of equivalence (eq) point are collected in Table 1. The relative error in accuracy 3, 22 of the Fe(+2) determination is equal to ;
Table 1. Some (e, Ee) pairs (e - end point) related to simulated, potentiometric titrations in the D+T system (full model).| Φe | EeV |
| 0.198 | 0.701 |
| 0.199 | 0.719 |
| 0.1998 | 0.661 |
| 0.1999 | 0.778 |
| 0.2 | 1.034 |
| 0.2001 | 1.365 |
| 0.2002 | 1.382 |
| 0.202 | 1.442 |
for e = eq = 0.2, one gets = 0.
The Fe+2, Fe+3 and Mn+2 ions form sulphate complexes, but sulphate complexes Mn(SO4)i+3-2i formed by Mn+3 ions are unknown in literature. However, the comparison of the curves obtained for pre-assumed stability constants of Mn(SO4)i+3-2i complexes presented in Figure 3 with the curves obtained experimentally 22 leads to conclusion, that the complexes – if exist – are relatively weak.
Figure 3.Fragments of hypothetical titration curves plotted for different pairs of stability constants (K31, K32) of the complexes Mn(SO4)i+3-2i : 1 (104, 107); 2 (103, 106); 3 (102.5, 105); 4 (102, 104); 5 (104, 0), 6 (103, 0); 7 (102, 0), 8 (0, 0); Mn(SO4)i+3 = K3iMn+3SO4i.
Final Comments
Redox systems are the most important and the most complex electrolytic systems. From thermodynamic viewpoint, the complexity of a redox system is expressed by the number and diversity of equilibrium constants involved in this system. The diversity is involved with the number of species, different types of reactions occurring between the species, and the number of elements involved in these species. The best thermodynamic approach to electrolytic systems is realizable according to the GATES principles, with none relevance to the stoichiometry of a chemical reaction, resulting from IUPAC recommendations 50. Within GATES and GATES/GEB in particular, the reaction notations are used only to formulate the expressions for the related equilibrium constants on the basis of mass action law. In this context, the paper is also designed as a reasonable proposal for consideration by IUPAC, in the next issues of ‘Color Books’ 51.
The correct formulation of dynamic redox systems was unknown in earlier (< 1994) literature, see 29, 30, 31. The obvious consequence of this fact was the lack of meaningful calculations, the effect of which would be a graphical representation of functional dependencies. The lack of the missing equation (i.e. GEB) caused that also charge balance (ChB) was completely omitted in the calculations, and pH = const during titrations was assumed. This assumption cannot withstand a criticism, not only in respect to titration of Br2 with NaOH 15, 16, 29, 30, 31, but also in the cases where the buffer capacity of the titrand is high, see e.g. 1, 2, 3. That ‘obligatory’ approach to the redox electrolyte systems has been repeatedly stigmatized in our papers cited above, and in 52, 53, 54. Simply, earlier approaches to formulation of redox systems, based on stoichiometry of reactions, are perceived as clumsy/invalid attempts to the problem in question.
GATES and GATES/GEB in particular, relies on the fact that the chemistry involved is predictable on the basis of knowledge of credible physicochemical data related to the species involved in the system in question.
The GEB plays a distinguishing role in redox systems; it is fully compatible with charge and concentration balances and, therefore, completes the set of equations necessary for thermodynamic solution of redox systems, of any degree of complexity, assuming that all relevant physicochemical knowledge is available. The results obtained according to GATES principles are also applicable for analytical purposes, especially in context with GEM 3, 23.
The identity (0 = 0) procedure applied to linear combination of equations relating to non-redox systems(see Eq. 5 in D and T), is far more convenient/efficient than the procedure based directly on the matrix calculations 55. The ‘shortest’ form (17a) of GEB related to the redox D+T system is different form identity. Then GEB for this system (e.g. Eq. 17a) is linearly independent from equations 7a, 9a, 10a, 14a, 15a. Then the linear dependency/ independency of 2f(O) - f(H) is confirmed here as the criterion distinguishing between non-redox and redox electrolytic systems. This regularity was also stated for all systems in amphiprotic solvents, and in mixed-solvent media.
In D and D+T, the core balance for f(SO4) is identical with elemental balance f(S). For comparison, in the non-redox system with oxalate and carbonate species involved, we can write separate balances for oxalates and carbonates. In the redox system, where oxalate is transformed into carbonate species, one common balance (for oxalate + carbonate) is needed 2.
If oxidation numbers for some elements constituting the system under consideration are unknown beforehand, the Approach II to GEB must be applied, because the prior knowledge of oxidation numbers is not needed there. Oxidation number is the redundant concept within GATES/GEB. Note that the oxidation number is the contractual term, and sometimes encounters fundamental difficulties, or atoms in organic compounds and species, with radicals and ion-radicals involved. It is one of the paramount advantages of the Approach II to GEB. It should be noticed that the oxidation number, representing the degree of oxidation of an element in a compound or a species is a contractual concept. Moreover, within the Approaches I and II to GEB, the roles of oxidants and reductants are not ascribed a priori to particular components forming the redox system and to the species formed in this system.
These basic properties of the balance 2∙f(O) – f(H) for redox systems were unknown in scientific world before 2005, and the linear independency/dependency of 2∙f(O) – f(H) as the fundamental/practical criterion distinguishing redox/non-redox systems of any degree of complexity was also unknown. Here is the hidden simplicity, which had to be discovered, as the Approach II to GEB. One of the authors (™) contends that the discovery of the Approach II GEB would most likely be impossible without the prior discovery of the Approach I to GEB. Any generalization presupposes belief in the unity and simplicity of Nature.
Instead of Epilogue
Further (loose) remarks concerning the words: card game, players, fans, money, debt of honor. Pure mathematics is, in its way, the poetry of logical ideas. One seeks the most general ideas of operation which will bring together in simple, logical and unified form the largest possible circle of formal relationships. In this effort toward logical beauty, new formulas are discovered, necessary for the deeper penetration into the laws of nature.
The art of reasoning is nothing more than a well-ordered language. The Renaissance posed the question of the criterion of determining whether the sign actually means what it meant, and the decision was provided by similarity. With the advent of the classical age, the question arose: how and how is the sign related to what it means? Classicism resolved the problem by offering an analysis of the representation, and the present day an analysis of the sense of meaning.
According to Aristotle, creating a good metaphor is tantamount to seeing similarities in dissimilar things. Metaphors can be alive or dead; living metaphors are revealing. Nevertheless, the best determinant of living metaphors is their uniqueness - which makes them so unusual and desired by their recipients. The paraphrase of such metaphors cannot exhaust the meaning that is the vehicle of innovation.
Pure mathematics is, in its way, the poetry of logical ideas. One seeks the most general ideas of operation which will bring together in simple, logical and unified form the largest possible circle of formal relationships. In this effort toward logical beauty spiritual formulas are discovered necessary for the deeper penetration into the laws of Nature (A. Einstein).
References
- 1.Michałowski T. (2011) . Application of GATES and MATLAB for Resolution of Equilibrium, Metastable and Non-Equilibrium Electrolytic Systems, Chap. 1: 1 – 34, in: Applications of MATLAB in Science and Engineering (ed. Michałowski T), InTech - Open Access publisher in the fields of Science, Technology and Medicine .
- 2.Michałowska-Kaczmarczyk A M, Spórna-Kucab A, Michałowski T. (2017) . Generalized Electron Balance (GEB) as the Law of Nature in Electrolytic Redox Systems, Chap. in:Redox: Principles and Advanced Applications (ed. Ali Khalid MA) .
- 3.Michałowska-Kaczmarczyk A M, Spórna-Kucab A, Michałowski T. (2017) . Principles of Titrimetric Analyses According to Generalized Approach to Electrolytic Systems (GATES), Chap. in:Advances in Titration Techniques, ed. Vu Dang Hoang, InTech .
- 4.Michałowska-Kaczmarczyk A M, Spórna-Kucab A, Michałowski T. (2017) A Distinguishing Feature. of the Balance 2∙f(O) – f(H) in Electrolytic Systems. The Reference to Titrimetric Methods of Analysis, Chap. in: Advances in Titration Techniques (ed. Vu Dang Hoang) .
- 5.Michałowska-Kaczmarczyk A M, Spórna-Kucab A, Michałowski T. (2017) Solubility products and solubility concepts. Descriptive Inorganic Chemistry. Researches of Metal Compounds. (ed. Akitsu T) InTech , Chap 5, 93-134.
- 6.Michałowski T, Pilarski B, Asuero A G.. Michałowska-Kaczmarczyk AM (2019) Modeling of Acid-Base Properties in Binary-Solvent Systems, Chap. 9.4: 665-690 in "Handbook of Solvents", Properties, (ed. Wypych G), ChemTec Publishing, 3rd Edition , Toronto .
- 7.Michałowska-Kaczmarczyk A M, Michałowski T. (2019) The new paradigm in thermodynamic formulation of electrolytic systems– a review. , Archive of Biomedical Science and Engineeringhttps://www.peertechz.com/articles/ABSE-5-113.pdf 5(1), 19-61.
- 8.Michałowska-Kaczmarczyk A M, Michałowski T. (2013) Comparative balancing of non-redox and redox electrolytic systems and its consequences. , American Journal of Analytical Chemistry
- 9.Michałowska-Kaczmarczyk A M, Michałowski T. (2019) General Properties of the Balance 2f(O)-f(H) in Electrolytic systems. Some Detailed Remarks on Elemental versus Core Balances. , Journal of Clinical Pharmacy 1(1), 5-16.
- 10.Michałowska-Kaczmarczyk A M, Spórna-Kucab A, Michałowski T. (2017) . General Properties of the Balances 2∙f(O)-f(H) Related to Electrolytic Systems, Analytical Chemistry: An Indian Journal .
- 11.Michałowska-Kaczmarczyk A M, Michałowski T. (2018) The Balance 2∙f(O) – f(H) as a Cornerstone in Formulation of Electrolytic Systems. , Journal of New Developments in 2(1), 1-13.
- 12.Michałowska-Kaczmarczyk A M, Spórna-Kucab A, Michałowski T. (2018) The balance 2∙f(O) – f(H) as a keystone in formulation of electrolytic systems. , Research and Reviews in Computational Chemistry
- 13.Michałowska-Kaczmarczyk A M, Michałowski T. (2018) The distinguishing role of 2.f(O) - f(H) in electrolytic systems. , Biomedical Journal of Scientific & Technical Research
- 14.Michałowska-Kaczmarczyk A M, Michałowski T. (2019) General Properties of the Balance 2f(O)-f(H) in Electrolytic systems. Some Detailed Remarks on Elemental versus Core Balances. , Journal of Clinical Pharmacy 1(1), 5-16.
- 15.Meija J, Michałowska-Kaczmarczyk A M, Michałowski T. (2017) Redox titration challenge, Analytical and Bioanalytical Chemistry.
- 16.Michałowski T, Michałowska-Kaczmarczyk A M, Meija J. (2017) Solution of redox titration challenge. , Analytical and Bioanalytical Chemistry 409(17), 4113-4115.
- 17.Michałowska-Kaczmarczyk A M, Spórna-Kucab A, Michałowski T. (2017) Formulation of simple electrolytic redox systems according to GATES/GEB principles. , Journal of Chemistry and Applied Chemical Engineering
- 18.Michałowska-Kaczmarczyk A M, Spórna-Kucab A, Michałowski T. (2017) A general property differentiating between redox and non-redox electrolytic systems and its consequences. , International Journal of Mathematics and Statistics Invention
- 19.Michałowska-Kaczmarczyk A M, Michałowski T. (2014) GATES as the Unique Tool for Simulation of Electrolytic Redox and Non-Redox Systems. , Journal of Analytical & Bioanalytical Techniques doi:, 10-4172.
- 20.Michałowska-Kaczmarczyk A M, Spórna-Kucab A, Michałowski T. (2017) Some Regularities Involved with Oxidation Numbers Stated in Formulation of Redox Systems According to GATES/GEB Principles. , Journal of Analytical, Bioanalytical and Separation Techniques
- 21.Michałowska-Kaczmarczyk A M, Spórna-Kucab A, Michałowski T. (2017) Oxidation number, oxidant and reductant as derivative concepts within GATES/GEB formulation. , Journal of Chemistry and Applied Chemical Engineering
- 22.Michałowski T. (1981) Some remarks on acid-base titration curves. , Chemia Analityczna 26, 799-813.
- 23.Michałowski T, Pietrzyk A, Ponikvar-Svet M, Rymanowski M. (2010) The Generalized Approach to Electrolytic Systems: II. The Generalized Equivalent Mass (GEM) Concept, Critical Reviews in Analytical Chemistry.
- 24.Michałowska-Kaczmarczyk A M, Michałowski T. (2020) . Justification of Generalized Electron Balance (GEB) as the Law of Nature (Editorial), Advances in Chemistry and Chemical Technology .
- 25.Michałowski T. (2014) Generalized electron balance (GEB) as a law of preservation for electrolytic redox systems. In: 65th Annual Meeting of the International Society of Electrochemistry , Lausanne, Switzerland .
- 26.Michałowska-Kaczmarczyk A M, Michałowski T. (2014) Generalized Electron Balance for Dynamic Redox Systems in Mixed-Solvent Media. , Journal of Analytical Sciences, Methods and Instrumentation 4(4), 102-109.
- 27.Pilarski B, Dobkowska A, Foks H, Michałowski T. (2010) Modelling of acid–base equilibria in binary-solvent systems: A comparative study. , Talanta
- 28.Michałowski T. (2002) Effect of Mutual Solubility of Solvents in Multiple Extraction. , Journal of Chemical
- 29.Michałowski T, Lesiak A. (1994) Acid-base titration curves in disproportionating redox systems. , Journal of Chemical Education
- 30.Michalowski T. (1994) Calculation of pH and potential E for bromine aqueous solutions. , Journal of Chemical Education 71(7), 560-562.
- 31.Michałowski T, Lesiak A. (1994) Formulation of generalized equations for redox titration curves, Chemia Analityczna (Warsaw)http://www.chem.uw.edu.pl/chemanal/.39:. 623-637.
- 32.Michałowski T, Wajda N, Janecki D. (1996) A Unified Quantitative Approach to Electrolytic Systems, Chemia Analityczna. , (Warsaw)
- 33.Michałowski T, Baterowicz A, Madej A, Kochana J. (2001) An extended Gran method and its applicability for simultaneous determination of Fe(II) and Fe(III), Analytica Chimica Acta.
- 34.Michałowski T, Rymanowski M, Pietrzyk A. (2005) Non-typical Brönsted’s acids and bases. , Journal of Chemical
- 35.Michałowska-Kaczmarczyk A M, Spórna-Kucab A, Michałowski T. (2017) Dynamic Buffer Capacities in Redox Systems. , Journal of Chemistry and Applied Chemical Engineering 1(2), 1-7.
- 36.Michałowski T, Parczewski A. (1978) A new definition of buffer capacity. , Chemia Analityczna 23, 959-964.
- 37.Michałowski T, A G. (2012) New approaches in modelling the carbonate alkalinity and total alkalinity, Critical Reviews in Analytical Chemistry.
- 38.Michałowska-Kaczmarczyk A M, Michałowski T. (2016) Application of Simms Constants in Modeling the Titrimetric Analyses of Fulvic Acids and Their Complexes with Metal Ions. , Journal of Solution Chemistry 45, 200-220.
- 39.Pilarski B, Michałowska-Kaczmarczyk A M, Asuero A G, Dobkowska A, Lewandowska M et al. (2014) A New Approach to Carbonate Alkalinity. , Journal of Analytical Sciences, Methods and Instrumentation 4, 62-69.
- 40.Michałowska-Kaczmarczyk A M, Michałowski T. (2020) Dynamic buffer capacityversusalkalinity. Formulation in terms of Simms constants idea. , Archive of Biomedical Science and Engineering 6(1), 1-9.
- 41.Michałowska-Kaczmarczyk A M, Michałowski T, Toporek M. (2016) Formulation of Dynamic Redox Systems according to GATES/GEB Principles. , International Journal of Electrochemical Science 11, 2560-2578.
- 42.Michałowski T. (2019) A dynamic redox system with ascorbic acid formulated and simulated according to GATES/GEB principles. , Current Topics in Analytical Chemistry 11, 31-41.
- 43.Michałowska-Kaczmarczyk A M, Michałowski T. (2020) Formulation of metastable redox systems according to GATES/GEB principles. Simulated Ascorbinometric Titration of Ferricyanide. , Acta Scientific Biotechnology 1(2), 1-8.
- 44.Marx D, Tuckerman M E, Hutter J, Parrinello M. (1999) The nature of the hydrated excess proton in water. , Nature 397, 601-604.
- 45.Asuero A G, Michałowski T. (2011) Comprehensive formulation of titration curves referred to complex acid-base systems and its analytical implications. , Critical Reviews in Analytical Chemistry 41(2), 151-187.
- 46.Michałowski T, Asuero A G, Ponikvar-Svet M, Toporek M, Pietrzyk A et al. (2012) Principles of computer programming applied to simulated pH–static titration of cyanide according to a modified Liebig-Denigès method. , Journal of Solution Chemistry 41(7), 1224-1239.
- 48.Ignatov Mosin OV. (2015) . Structural Models of Water and Ice Regarding the Energy of Hydrogen Bonding, Nanotechnology Research and Practice 7(3), 96-118.
- 49.Michałowska-Kaczmarczyk A M, Rymanowski M, Asuero A G, Toporek M, Michałowski T. (2014) Formulation of Titration Curves for Some Redox Systems. , American Journal of Analytical Chemistry 5, 861-878.
- 50.Inczédy J, Lengyel T. International Union of Pure and Applied Chemistry, Blackwell Scientific Publications (1997) Ure AM Compendium of Analytical Nomenclature, Definitive Rules.
- 51.Color Bookshttps://iupac.org/what-we-do/books/color-books/,https://ro.pinterest.com/danadamonica/iupac-color-books/.
- 52.Michałowska-Kaczmarczyk A M, Asuero A G, Michałowski T. (2015) Why not stoichiometry” versus “Stoichiometry – why not?” Part I. General context, Critical Reviews in Analytical Chemistry.
- 53.Michałowska-Kaczmarczyk A M, Asuero A G, Toporek M, Michałowski T. (2015) Why not stoichiometry” versus “Stoichiometry – why not?” Part II. GATES in context with redox systems, Critical Reviews in Analytical Chemistry.
Cited by (4)
This article has been cited by 4 scholarly works according to:
Citing Articles:
Journal of Biomedical Research & Environmental Sciences (2021) Crossref
Journal of Biomedical Research & Environmental Sciences (2021) OpenAlex
Journal of Experimental Biology and Agricultural Sciences (2020) Crossref
Journal of Experimental Biology and Agricultural Sciences (2020) OpenAlex
