Abstract
Obesity is associated with a number of serious medical complications, which are often referred to as the “insulin resistance syndrome”. The aim of the present study was performed to investigate the possible interaction between a conventional drug used for management of cholesterol and traditional herbal remedies on the obesity. This was carried through out: through estimation of blood test; Estimation of serum tests; Determination of oxidative stress biomarkers and the antioxidant enzymes activities in the liver were assayed; Histopathological examination of the liver and kidney of adult male albino rats were done. In the present study, the serum levels of the total protein and albumin in the obesity group (7.1± 0.2) and (4.78 ± 0.19); respectively were significantly (p ≤ 0.05) more than those of the control group (6.5±0.1) and (3.95± 0.1).The administration of (fennel group) revealed significant (P<0.05) decrease in the serum levels of the albumin and total protein (4.38± 0.1) and (6.65± 0.2); respectively as compared to the obesity group (4.78 ± 0.19) and (7.1± 0.2(. The total cholesterol of the group(5) (fennel and ator) after two weeks from a high fat diet than treatment with fennel and Ator through six weeks equal 142.86±5.9, 100.4±8.68, 93.29±5.99, 87.1±11.28, 80.4±21.55, 78.1±6.7 and 77.1±6.87; respectively. The present study showed a significant (P<0.05) increase in the activities of ALT, AST and ALP in the obesity group which recorded as (60.5±11.45), (57.25±6.3) and (845.0±49.47); respectively as compared to the control group (28.25±1.7), (38.5±3.87) and (537.0±41.5); respectively. The fennel group caused significant decrease in the activities of these enzymes (41.0± 2.9), (42.25+3.2) and (717.75+48.6); respectively compared to the obesity group. Ator group showed a significant decrease in the activities of these enzymes (40.0±2.16), (42.5±3.1) and (679.25±41.16); respectively compared as obesity group. The activity of AlT, AST and ALP in the fennel and ator group (32.75±2.5), (40.5±2.38) and (601.25±17.5); respectively were near to the control group.
Author Contributions
Academic Editor: Qianqian Song, Wake Forest School of Medicine, Wake Forest Baptist Comprehensive Cancer Center, Medical Center Boulevard, Winston-Salem, NC 27157, United States.
Checked for plagiarism: Yes
Review by: Single-blind
Copyright © 2019 Nawal A. Elghazaly, et al.
 This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Competing interests
The authors have declared that no competing interests exist.
Citation:
Introduction
The most common complications involve alterations in metabolic function that are risk factors for cardiovascular disease, namely hyperglycemia, hyper-insulinemia, and glucose intolerance, liver fat accumulation, dyslipidemia (increased serum triglyceride and decreased serum HDL-cholesterol concentrations; a predominance of smaller LDL particles and an increased number of large VLDL particles), and endothelial dysfunction 1. Fat accumulation in non-adipose tissues such as in the liver, skeletal muscles, heart appears to be a key feature distinguishing metabolically healthy from metabolically abnormal subjects. Obesity has deleterious effects on metabolic homeostasis, and affects numerous body organs 2, 3. Lipids are essential for membrane synthesis, maintenance of membrane integrity, as an energy source, as hormone precursors, and as signaling molecules. Hyperlipidemia is usually characterized by elevated serum total cholesterol and decrease in High density lipoprotein (HDL) cholesterol and increase in Low density lipoprotein (LDL) cholesterols. It is observed that there is also an association between hyperlipidemia, and serum triglycerides 4. In the presence of severe hypertriglyceridemia or mixed hyperlipidemia, the combination of statins and fibrates is a possible treatment 5. Fennel is an aromatic herb belonging to the Parsley family. It is used as a spice and possesses a sweet taste that is similar to anise. Fennel seed shape is oval and has a strong scent, while the fruit have a slightly sweet and slightly spicy. Sweet fennel oil is made up predominantly of anethole (50 to 80%), fenchone, and estragole. The seeds also contain fiber and complex carbohydrates 6.
Material and Methods
Experimental animals and treatment: Adult male Albino rats weighing 150–160gm were obtained from the animal house of the Faculty agriculture, Alexandria University, Egypt. They were housed in a clean and well ventilated animal house with a constant 12 hr light and 12 hr dark schedule. They lived in five different groups (seven animals per group) at 25-27oC.The animals were provided with standard diet and tap water was supplied. They were acclimatized under laboratory conditions for one week before the experiment. Diets; Composition of the experimental diet (g/kg diet) was according to the formula of 7. It is including the normal diet for the control rats (fat 5%, carbohydrates 65%, proteins 20.3%, fiber 5%, salt mixture 3.7% and vitamin mixture 1%). The high fat diet contained fat 46%, carbohydrates 24%, proteins 20.3%, fiber5%, salt mixture 3.7% and vitamin mixture 1%.Normal and HFD; high fat diet constituents were purchased from El-Gomhoria Company, Cairo, Egypt. HFD was preserved at 4oC until being used. Atorvastatin was obtained from local pharmacies, Cairo, Egypt and ground using a mortar. The drugs were administered orally once a day. Fennel (Foeniculum vulgare) seeds were obtained and dried fennel seeds (Shamar) were washed with tap water to remove possible potential dust. Afterwards, it was dried by cotton cloth to remove the excess liquid prior to drying. Drying was achieved at room temperature for 48 hr. Then a grinder mill and sieves were used to obtain a powder particle size of less than 0.2mm 8.
Experimental design: Thirty five Male Albino rats were randomly divided into five groups; (7 rats/ group) as follows: Group 1: (control group): Animals of this group were fed normal diet. Group 2: (obese group): rats of this group were fed high fat diet for two weeks. It is including the normal diet for control rats (fat 5%, carbohydrates 65%, proteins 20.3%, fiber 5%, salt mixture 3.7% and vitamin mixture 1%). The high fat diet contained fat 46%, carbohydrates 24%, proteins 20.3%, fiber 5%, salt mixture 3.7% and vitamin mixture 1%.Normal and HFD constituents were purchased from El-Gomhoria Company, Egypt. HFD was preserved at 4oC until used. Group 3: (obese rats treated with fennel): Animals of this group were fed high fat diet; HFD for two weeks then treated with the fennel herb fennel 300 mg/kg body weight. Group 4: (obese rats treated with atorvastatin drug): rats of this group were received high fat diet two weeks then treated with the atorvastatin drug 10 mg/kg 9. Group 5: (obese rats treated with atorvastatin drug and fennel herb): rats of this group were received high fat diet for two weeks then treated with atorvastatin drug and fennel herb.
The rats received a high fat diet contained containing fat 46%, carbohydrates 24%, proteins 20.3%, fiber 5%, salt mixture 3.7% and vitamin mixture 1%. Normal and HFD constituents were purchased from El-Gomhoria Company, Egypt. HFD was preserved at 4oC until used for two weeks. Treatment with fennel and atorvastatin drug started after two weeks. Studies of blood samples: For the determination of blood parameters, some of blood was collected into Ethylenediaminetetraacetic acid (EDTA) EDTA –treated tubes for determination hematological parameters as: Determination of serum total protein was done according to Gornall et al. 10. Determination of serum albumin was done according to Doumas et al.11. Determination of serum total cholesterol was done according to Allain et al. 12. Determination of serum triglycerides was done according to Fassati and Prencipe 13. Determination of serum high density lipoproteins (HDL) Cholesterol levels and Determination of serum low density lipoproteins (LDL) Cholesterol levels according to Fruchart 14. Determination of serum urea was done according to Fawcett and Soctt 15. Determination of serum creatinine was done according to Bartles et al.16. Determination of alanine aminotransferase(ALT) and Determination of aspartate aminotransferase activity (AST) according to Tietz 17. Determination of alkaline phosphatase (ALP) was done according to DGKC 18. Determination of lipid peroxide (Malondialdehyde) (MDA) was done according to Ohkawa et al. 19. Determination of Myeloperoxidase (MPO) was done according to Zhang et al.20. Determination of Catalase (CAT) was done according to Aebi 21. Determination of Superoxide dismutase (SOD) was done according to Nishikimi et al.22. Determination of Glutathione peroxide (GPx) was done according to Paglia and Valentine 23.
Histopathological Examination of Liver, kidney and Heart Tissues
Conventional techniques of Paraffin wax sectioning and Haematoxylin and Eosin staining were used for histological studies 24. Immediately pieces of each tissue were fixed in formalin 10% solution for 12 hours, dehydrated through ascending grades of ethyl alcohol until they reached the absolute alcohol (1 hour), they were then transferred to xylol. Tissues were placed in a mixture of melted wax and xylol (1:1) for about 10 minutes and transferred to paraffin wax 56ċ (3 changes for about 2 hours). For sectioning, the paraffin blocks were mounted in a microtome where successive sections adhere to form a straight ribbon. A drop of distilled water was spread on clean slides on which a small section of the paraffin ribbon was float. The slide was hold high up over a Bunsen burner flame till the sections flatten out and firmly adhere to the slide. Slides were immersed in xylol for three minutes to dissolve the paraffin and then transferred to absolute alcohol for one minute to remove the xylol, sections were dehydrated by passing them down 96%, 90%, 80%, 70% and 50% alcohol, Haematoxylin counter stained by eosin. Sections were examined for histopathological changes. Statistical Analysis using ANOVA test, Significance between groups was done using Post Hoc Test (LSD), statistically significant at p ≤ 0.05.
Result
Effect of the Fennel and Ator on the Body Weight of Obese Male Albino Rats
It was found from data of Table 1, that the body weight of the group (2), group (3), group (4) and group (5) after two weeks form high fat diet (229.29±3.9), (224.29±10.4), (222.4±15.7) and (218.29±4.39) significant increase than group (1) (control group) (198.29±11.3). The body weight of these rats at one week after treatment in Table 1 was reported as; 190.0±9.98, 228.14±18.7, 233.86±17.18, 225.0±25.8 and 240.57±10.66; respectively, whereas, group (5) (fennel and ator) is significantly increased than group (2) (obesity), group (3) (fennel) and group (4) (ator). As shown in Table 1, a significant increase was observed in body weight value (232.0±3.06) in obesity rats as compared to the control group (205.14±17.63). However; (gp3), (gp4) supplementation was able to recover this elevation (216.7±10.98) and (211.4±24.78) respectively after two weeks from treatment. But group (5) (207.4±10.36) recover this elevation to the normal value.
After three weeks from treatment with fennel and ator, it was found that the body weight of the group(5) (203.0±11.4) is significantly decreased than group (2), group (3) and group (4) which recorded in Table 1 as (241.7±25.79), (211.29±8.46) and (205.0±25.99); respectively. Group (3) and group (4) showed a significant decrease than group (2). The body weight after four weeks from treatment of fennel and ator which indicated that the body weight of obese rats (279.57±5.5) had increased more than the control group (212.57±17.2). On the other direction, group (3), group (4) and group (5) were able to recover this elevation as (206.4±8.66), (199.7±26.5) and (194.57±10.97); respectively. Group (5) was able to reach the normal value of the control group. In Table 1 the body weight of these rats at five weeks from treatment was reported as; 215.57±19.25, 284.29±4.79, 198.57±6.55, 194.7±26.0 and 191.0±6.9; respectively, which indicated that the treatment with fennel and ator group (5) more effecting on the body weight than treatment with fennel only group (3) or ator only group(4). The rats which were treated with fennel and ator after six weeks were recorded in Table 1 as (184.29±8.38) significant decrease as compared to the group (3) and group (4) (193.14±6.67) and (188.43±25.15); respectively. Group (3) and group (4) significantly decreased more than group (2) which was recorded as (289.57±7.46) and group (2) significantly increased compared to the control group (230.1±25.97).
Table 1. Effect of Fennel and Ator on the body weight of obese male albino rats| No of weeks | Control(gp1) | Obesity(gp2) | Fennel (gp3) | Ator (gp4) | Fennel+Ator (gp5) | LSD(5%) |
| After two weeks form | 198.29b±11.31 | 229.29a±3.90 | 224.29a±10.42 | 222.43a±15.71 | 218.29a ± 4.39 | 11.11 |
| high fat diet | ||||||
| One week form treatment | 190.0b ± 9.98 | 228.14a±18.74 | 233.86a± 17.18 | 225.0a ± 25.83 | 240.57a±10.61 | 19.077 |
| Two weeks from treatment | 205.14b±17.63 | 232.0a ± 3.06 | 216.71ab±10.98 | 211.43b±24.78 | 207.43b± 0.36 | 16.641 |
| three weeks from | 209.0b ± 16.90 | 241.71a±25.79 | 211.29b ± 8.46 | 205.0b ± 25.99 | 203.0b ± 1.42 | 20.874 |
| treatment | ||||||
| Four weeks from treatment | 212.57b ± 17.2 | 279.57a ± 5.53 | 206.43bc ± 8.66 | 199.71bc±26.5 | 194.57c± 0.97 | 17.083 |
| Five weeks from treatment | 215.57b±19.25 | 284.29a ± 4.79 | 198.57c ± 6.55 | 194.71c± 26.02 | 191.0c ± 6.93 | 16.64 |
| Six weeks from treatment | 230.14b±25.97 | 289.57a ± 7.46 | 193.14c ± 6.67 | 188.43c± 25.15 | 184.29c ± 8.38 | 18.763 |
Effect of Fennel and Ator on Serum Total Protein Level and Albumin level of obese Male Albino Rats
The serum levels of the total protein and albumin in the obesity group (7.1± 0.2) and (4.78 ± 0.19); respectively were significantly (p ≤ 0.05) more than those of the control group (6.5±0.1) and (3.95± 0.1); Table 6. While, the administration of (fennel group) revealed a significant (P<0.05) decrease in the serum levels of the albumin and total protein (4.38± 0.1) and (6.65± 0.2); respectively as compared to the obesity group (4.78 ± 0.19) and (7.1± 0.2(. Table 2
Table 2. Effect of Fennel and Ator on serum total protein level and albumin level of obese male albino rats:| Parameters | Control(gp1) | Obesity(gp2) | Fennel (gp3) | Ator (gp4) | Fennel +Ator | LSD |
| (gp5) | -5% | |||||
| Total protein (g/dl) | 6.50b+ 0.13 | 7.10c+ 0.22 | 6.65b+0.24 | 6.63b+0.22 | 6.53a+0.10 | 0.253 |
| Albumin (g/dl) | 3.95d ± 0.13 | 4.78a ± 0.19 | 4.38b ± 0.10 | 4.28bc±0.17 | 4.15cd ± 0.13 | 0.221 |
Effect of Fennel and Ator on the Cholesterol Level of the Obese Rats
Table 3 showed cholesterol of the five experimental groups of animals after two weeks form recieving a high fat diet then treatment with fennel and Ator through six weeks. Total cholesterol of the group(5) (fennel and ator) after two weeks form a high fat diet than treatment with fennel and Ator through six weeks equal 142.86±5.9, 100.4±8.68, 93.29±5.99, 87.1±11.28, 80.4±21.55, 78.1±6.7 and 77.1±6.87; respectively. It is indicating a decrease in the cholesterol and reaching to the normal control level which was recorded in Table 5 as 78.0±52.2, 78.7±53.48, 84.4±42.1, 89.86±11.5, 97.1± 36.59, 105.0±8.0 and 106.86±7.65; respectively. The cholesterol of group(3) (fennel) indicated in Table 5 as 141.7±5.96, 118.1±5.76, 111.7±10.06, 101.0± 12.15, 92.57±36.66, 88.86±11.16 and 85.4±8.8 as compared to the group (4) (ator group) 145.0±6.78, 118.29±12.85, 97.29±20.1, 91.57±24.4, 85.1±9.96, 79.4±7.4 and 78.1±7.78 showed no significance form each other but significantly decreased more than group(2) (obesity group) which the total cholesterol 144.1±8.49, 145.57±6.78, 149.1±5.58, 154.4±21.88, 166.1±17.1, 174.4±31.95 and 178.0±31.3 increase more than the control group.
Table 3. Effect of Fennel and Ator on cholesterol of obese rats| Parameters Cholesterol (mg/dl) | Control (gp1) | Obesity(gp2) | Fennel (gp3) | Ator (gp4) | Fennel+Ator (gp5) | LSD (5%) |
| After two week form high fat diet | 78.0b ± 52.23 | 144.14a±8.49 | 141.71a ± 5.96 | 145.0a ± 6.78 | 142.86a ± 5.90 | 26.365 |
| One week form treatment | 78.71c±53.48 | 145.57a±6.78 | 118.14ab ±5.76 | 118.29ab±12.85 | 100.43bc ±8.68 | 27.531 |
| Two weeks from treatment | 84.43c 42.11 | 149.14a±5.58 | 111.71b±10.06 | 97.29bc ± 20.11 | 93.29bc ± 5.99 | 23.645 |
| three weeks from treatment | 89.86b±11.54 | 154.43a±21.88 | 101.0b ± 12.15 | 91.57b ± 24.42 | 87.14b ± 11.28 | 18.799 |
| Four weeks from treatment | 97.14b±36.59 | 166.14a±17.14 | 92.57 b± 36.66 | 85.14b ± 9.96 | 80.43b ± 21.55 | 29.045 |
| Five weeks from treatment | 105.0b± 8.04 | 174.4a ±31.95 | 88.86bc ±11.16 | 79.43c ± 7.44 | 78.14c ± 6.72 | 17.671 |
| Six weeks from treatment | 106.86b ±7.65 | 178.0a ± 31.33 | 85.43c ± 8.81 | 78.14c ± 7.78 | 77.14c ± 6.87 | 17.087 |
Effect of Fennel and Ator on Triglyceride of Obese Rats
Table 4showed triglyceride of the five experimental groups of animals after two weeks form having a high fat diet then treatment with fennel and Ator through six weeks. Triglyceride of the group (5) (fennel and ator) after two weeks form having a high fat diet than treatment with fennel and Ator through six weeks equal 228.1± 33.8, 186.4±37.29, 173.57±24.09, 153.29±34.99, 130.4±32.47, 121.4±19.28 and 120.57±19.18; respectively. Indicating a decrease in the triglyceride and reach to the normal control which was recorded in Table 4 as 100.4±9.95, 102.29±7.2, 110.29±10.8, 124.1±37.0, 127.29±31.1, 131.1±25.6 and 135.1±27.39; respectively. The triglyceride of group(3) (fennel) indicated in Table 4as 225.7± 32.98, 196.86±40.77, 189.57±38.3, 165.7±34.5, 136.29±37.3, 126.57±35.68 and 125.7±35.26 as compared to the group (4) (ator group) 229.4±26.15, 195.4±40.85, 182.0±26.6, 165.0±11.28, 135.86±39.26, 125.4±13.3 and 123.29±13.7 showed no significance form each other but significant decrease than group(2) (obesity group) which the triglyceride 239.7±40.67, 300.86±13.8, 308.4±37.6, 315.29±45.6, 327.0±49.0, 341.29±28.7 and 345.0±27.0 increased more than the control group.
The triglyceride level of group (3) (fennel) indicated in Table 4 as 31.57±2.7, 32.57±4.58, 33.57±4.7, 36.4±7.09, 37.7±6.68, 37.57±8.5 and 38.57±7.76 as compared to the group (4) (ator group) 33.86±3.29, 34.29±5.68, 35.57±4.4, 37.0± 3.5, 38.1±9.1, 39.29±3.3 and 39.57±1.6 showed no significant form each other but significant increase than group(2) (obesity group) which the high density lipoprotein (HDL) 37.0±6.3, 31.29±4.35, 28.57±7.0, 28.29±6.78, 27.7±5.15, 26.29± 4.6 and 24.1±2.5 decrease than the control group.
Table 4. Effect of Fennel and Ator on triglyceride of obese rats| Parameters; Triglyceride(mg/dl) | Control (gp1) | Obesity (gp2) | Fennel (gp3) | Ator(gp4) | Fennel+Ator (gp5) | LSD (5%) |
| After two week form high fat diet | 100.43b±9.95 | 239.71a±40.67 | 225.71a±32.98 | 229.43a±26.2 | 228.14a±33.8 | 33.35 |
| One week form treatment | 102.29c±7.20 | 300.86a±13.80 | 196.86b±40.7 | 195.43b±40.8 | 186.43b±37.3 | 34.39 |
| Two weeks from treatment | 110.29c±10.81 | 308.43a±37.64 | 189.57b±38.3 | 182.0b±26.63 | 173.57b±24.09 | 31.97 |
| three weeks from treatment | 124.14c±37.02 | 315.29a±45.64 | 165.71b±34.5 | 165.0b±11.28 | 153.29bc±34.9 | 37.8 |
| Four weeks from treatment | 127.29b±31.14 | 327.0a ± 49.04 | 136.29b±37.3 | 135.86b±39.2 | 130.43b±32.47 | 41.89 |
| Five weeks from treatment | 131.14b±25.67 | 341.29a±28.74 | 126.57b±35.6 | 125.43b±13.3 | 121.43b±19.28 | 28.07 |
| Six weeks from treatment | 135.14b±27.39 | 345.0a ± 27.02 | 125.71b±35.2 | 123.29b±13.7 | 120.57b±19.18 | 27.96 |
Effect of Fennel and Ator on High Density Lipoprotein (HDL) of obese Rats
Table 5 showed high density lipoprotein (HDL) of the five experimental groups of animals after two weeks form having a high fat diet then treatment with fennel and Ator through six weeks. High density lipoprotein (HDL) of the group(5) (fennel and ator) after two weeks form having a high fat diet then treatment with fennel and Ator through six weeks equal 34.29±5.99, 35.86±4.45, 36.86±3.4, 37.7±6.1, 39.1±7.4, 40.29± 7.7 and 42.0±6.5; respectively, it is indicating an increase in the high density lipoprotein (HDL) and reaching to the normal control level which was record in Table 5 as 46.86±8.28, 46.29±9.0, 44.57±10.9, 43.29±7.67, 42.4±8.8, 42.0±11.96 and 42.0±11.1; respectively.
Table 5. Effect of Fennel and Ator on high density lipoprotein (HDL) of obese rats:| Parameters; High Density lipoprotein (HDL)(mg/dl) | Control (gp1) | Obesity (gp2) | Fennel (gp3) | Ator (gp4) | Fennel+Ator (gp5) | LSD(5%) |
| After two weeks form high fat diet | 46.86a ±8.28 | 37.0b ± 6.32 | 31.57b ± 2.70 | 33.86b ± 3.29 | 34.29b ± 5.99 | 6.2229 |
| One week form treatment | 46.29a ±9.01 | 31.29b ± 4.3 | 32.57b ± 4.58 | 34.29b ± 5.68 | 35.86b ± 4.45 | 6.4239 |
| Two weeks from treatment | 44.57a±10.91 | 28.57c ± 7.0 | 33.57bc ± 4.72 | 35.57bc ± 4.43 | 36.86b ± 3.44 | 7.2677 |
| three weeks from treatment | 43.29a ± 7.67 | 28.29b ± .78 | 36.43a ± 7.09 | 37.0a ± 3.51 | 37.71a ± 6.13 | 6.9899 |
| Four weeks from treatment | 42.43a ± 8.81 | 27.71b ±5.15 | 37.71a ± 6.68 | 38.14a ± 9.14 | 39.14a ± 7.40 | 8.2713 |
| Five weeks from treatment | 42.0a ± 11.96 | 26.29b± 4.61 | 37.57a ± 8.54 | 39.29a ± 3.30 | 40.29a ± 7.72 | 8.5632 |
| Six weeks from treatment | 42.0a ± 11.12 | 24.14b± 2.54 | 38.57a ± 7.76 | 39.57a ± 1.62 | 42.0a± 6.53 | 7.4953 |
Effect of Fennel and Ator on Low Density Lipoprotein (LDL) and Ratio (holesterol/HDL) of Obese rats
A significant increase was obCserved in serum low density lipoproteins LDL and Ratio cholesterol/HDL levels in obesity rats reaching a value of (151.57±29.88) and (7.3±1.59) respectively in compare with the control group (62.86±12.1) and (2.69± 0.81); respectively. Fennel treatment in combination with obesity group improved these elevations to be (44.7±12.96) and (2.29±0.5). Also treatment with ator drug gave a significant decrease these parameters (36.4±8.4) and (1.98±0.2) as compared to the obesity group. The treatment with fennel and ator showed a significant decrease in these parameters (33.1±5.15) and (1.85±0.2) compared to group (3) and group (4). Table 6
Table 6. Effect of fennel and Ator on low density lipoprotein (LDL) and (cholesterol/HDL ratio) of obese rats| Low density lipoproteins (LDL)(md/dl) | 62.86b±12.14 | 151.57a±29.88 | 44.71c ± 12.96 | 36.43c ±8.42 | 33.14c ±5.15 | 65.279* (<0.001*) |
| Ratio (cholesterol/HDL) | 2.69b ± 0.81 | 7.31a ± 1.59 | 2.29b ± 0.50 | 1.98b ± 0.24 | 1.85b ± 0.20 | 52.692* (<0.001*) |
Obesity administration was associated with a highly significant increase in urea level reaching (43.25±4.65) as compared to the control group (27.75±2.6). Treatment with fennel, ator, fennel with ator following obesity administration exhibited a high drop in this elevation to (30.5±3.1), (31.0±3.16) and (28.0±2.16); respectively as compared to the obesity group and this indicated that treatment with fennel with ator was near to the control value (27.75±2.6). Fennel group and ator group supplement successes partially in improving the creatinine level to be (1.0±0.09), (0.9±0.06); respectively in comparing with the obesity group (0.58+0.07). Although fennel and ator group indicated significant more decrease in creatinine level (0.78±0.0) as compared to group(3) and group(4) but increase than the control ( 0.58±0.07). The Uric acid level showed a significant increase, reaching (3.8±0.25) in obesity group as compared as to the control value (2.18+0.28).The administration of fennel, ator and fennel with ator for six weeks resulted in a partial improvement in the uric acid level (2.8±0.2), (2.98±0.2) and (2.68±0.28) as compared to the obesity group. In the opposite direction, the uric acid level of group (5) showed significant decrease comparing to group (3) and group (4). Table 7
Table 7. Effect of fennel and ator on serum kidney function tests in obese rats| Parameters | Control(gp1) | Obesity(gp2) | Fennel (gp3) | Ator (gp4) | Fennel+Ator (gp5) | LSD (5%) |
| Urea (mg/dl) | 27.75b ±2.63 | 43.25a ±4.65 | 30.50b ± 3.11 | 31.0b ± 3.16 | 28.0b ± 2.16 | 4.8992 |
| Creatinine (mg/dl) | 0.58d ± 0.07 | 1.40a ± 0.14 | 1.02b ± 0.09 | 0.93b ± 0.06 | 0.78c ± 0.03 | 0.1322 |
| uric acid (mg/dl) | 2.18c ± 0.28 | 3.83a ± 0.25 | 2.80b ± 0.22 | 2.98b ± 0.22 | 2.68b ± 0.28 | 0.3753 |
Effect of Fennel and Ator on Serum Liver Function Tests of Obese Rats
Table 8 showed a significant (P<0.05) increases in the activities of ALT, AST and ALP in the obesity group which recorded as (60.5±11.45), (57.25±6.3) and (845.0±49.47); respectively as compared to the control group (28.25±1.7), (38.5±3.87) and (537.0±41.5); respectively. Administration of fennel group caused significant decreases in the activities of these enzymes (41.0± 2.9), (42.25+3.2) and (717.75+48.6); respectively compared to the obesity group. Ator group showed significant decreases in the activities of these enzymes (40.0±2.16), (42.5±3.1) and (679.25±41.16); respectively compared as obesity group. The activities of ALT, AST and ALP in the fennel and ator group (32.75±2.5), (40.5±2.38) and (601.25±17.5); respectively were near to the control group.
Table 8. Effect of fennel and ator on serum liver function tests in obese rats| Parameters | Control (gp1) | Obesity (gp2) | Fennel (gp3) | Ator(gp4) | Fennel+Ator(gp5) | LSD(5%) |
| alanine aminotransferase (ALT) (U/L) | 28.25c±1.71 | 60.50a± 11.45 | 41.0b+ 2.94 | 40.0b+ 2.16 | 32.75bc+ 2.50 | 8.3508 |
| aspartate aminotransferase activity (AST) (U/L) | 38.50b+3.87 | 57.25a+6.34 | 42.25b+3.20 | 42.50b+3.11 | 40.50b+2.38 | 6.06 |
| alkaline phosphatase (ALP) (U/L) | 537.0d+41.50 | 845.0a+49.47 | 717.75b+48.62 | 679.25b+41.16 | 601.25c+17.50 | 62.264 |
Effect of Fennel and Ator on Oxidative Stress Biomarkers of Obese Rats
Obesity was associated with a highly significant increase in MDA and MPO concentration reaching (52.75±6.8) and (55.5±6.19) respectively as compared to the control group (19.5±2.08) and (28.5±3.1). Treatment with (fennel), (ator) following obesity administration exhibited a drop in this elevation to (44.0±5.35), 43.75±2.6), (41.75±2.5), (42.75±4.99); respectively as compared to the obesity group .But the treatment with fennel and ator exhibited a high drop in this elevation to (35.0±3.7) and (39.75±1.7) as compared to group (3) and group (4). Table 9
Table 9. Effect of fennel and ator on Oxidative stress biomarkers in obese rats| Parameters | Control (gp1) | Obesity (gp2) | Fenunel (gp3) | Ator (gp4) | Fennel +Ator (gp5) | LSD (5%) |
| Malondialdehyde (MDA) | 19.50d±2.08 | 52.75a±6.80 | 44.0b±5.35 | 41.75b±2.50 | 35.0c±3.74 | 6.7234 |
| Myeloperoxidase (MPO) | 28.50c±3.11 | 55.50a±6.19 | 43.75b±2.63 | 42.75b±4.99 | 39.75b±1.71 | 6.1314 |
Effect of Fennel and Ator on Antioxidant Enzymes of Obese Rats
A significant decrease was observed in liver CAT, SOD and GPx level in obesity rats reaching a value of (29.5±2.08), (31.0±3.56) and (28.5±2.65); respectively compared to the control group (49.25±3.3), (63.5±3.87) and (57.25±6.6); respectively. Treatment with fennel in combination with obesity group partially improved this decrease in CAT, SOD and GPx level to be (38.5±1.29), (43.25±2.75) and (38.75±4.27); respectively and treatment with ator drug showed a significant increase in these enzymes (36.5±3.7), (46.5±5.8) and (41.0±2.8) as compared to the obesity group. While the group which treated with fennel and ator together showed significantly more increase in these enzymes(43.0±3.56), (49.75±4.9) and (44.0±3.7) as compared with group (3) and group(4) but not reach to the control value. Table 10
Table 10. Effect of fennel and ator on antioxidant enzymes in obese rats:| Parameters | Control(gp1) | Obesity (gp2) | Fennel (gp3) | Ator (gp4) | Fennel +Ator (gp5) | LSD (5%) |
| Catalase (CAT) | 49.25a±3.30 | 29.50d±2.08 | 38.50c±1.29 | 36.50c±3.70 | 43.0b±3.56 | 4.43 |
| Superoxide dismutase (SOD) | 63.50a±3.87 | 31.0c±3.56 | 43.25b±2.75 | 46.50b±5.80 | 49.75b±4.92 | 6.5 |
| Glutathione peroxide (GPx) | 57.25a±6.60 | 28.50c±2.65 | 38.75b±4.27 | 41.0b±2.83 | 44.0b 3.74 | 6.42 |
Effect of Ator, Fennel and Their Combination on the Histology of the kidney of the Obese Rats
The histological examination of the kidney of the control rats fed on a standard diet showing normal rounded capsules with normal Bowman's glomeruli, round proximal tubules and elongated distal tubules with high cuboidal cells figure 1. The rats fed (HFD) showed fatty degeneration of the tubules with eosinophilic material deposition, glomerular atrophy with wide urinary space and distal tubules with extrusion of nuclei into lumen figure 2. By comparison kidneys of rats which were treated by fennel after being obese and the control rats observed partial improvement in both Bowman's capsules and proximal tubules. Note the distal tubules show less focal fatty infiltration figure 3.While examination of rats kidney that were treated by ator after obesity showed improvement in Bowman's capsules with normal glomerular and partial improvement in proximal tubules and distal tubules, figure 4. Kidney of rats that were treated by fennel and ator after obesity and the control rats showed high improvement in the tissues with normal glomerular and that Most of Bowman's capsules and renal tubules, restoring their normal appearance figure 5.
Figure 1.Photomicrogragh of kidney section of control rat showing normal rounded capsules with normal Bowman,s glomeruli, round proximal tubules and elongated distal tubules with high cuboidal cells , (H&E) (40X).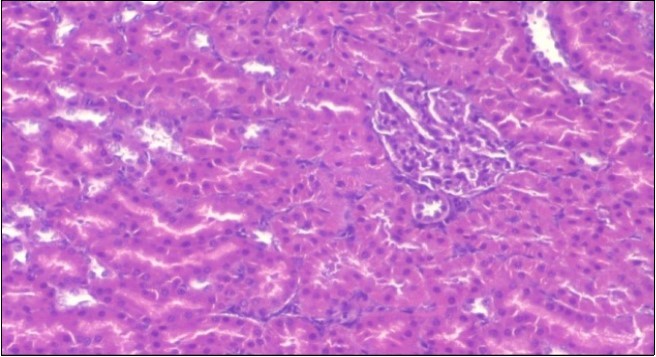
Figure 2.Photomicrogragh of kidney section of obese rat showing fatty degeneration of the tubules with eosinophilic matrial deposition, glomrerular atrophy with wide urinary space and distal tubules with extrusion of nuclei into lumen , (H&E) (40X).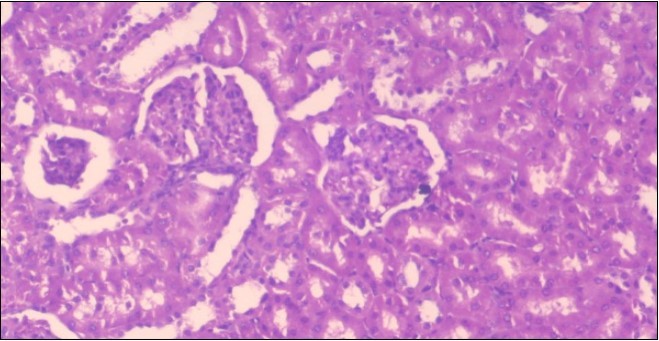
Figure 3.Photomicrogragh of kidney section of treated rat with fennel herb showing partial improvement in both Bowman's capsules and proximal tubules. Note the distal tubules show less focal fatty infiltration, (H&E) (40X)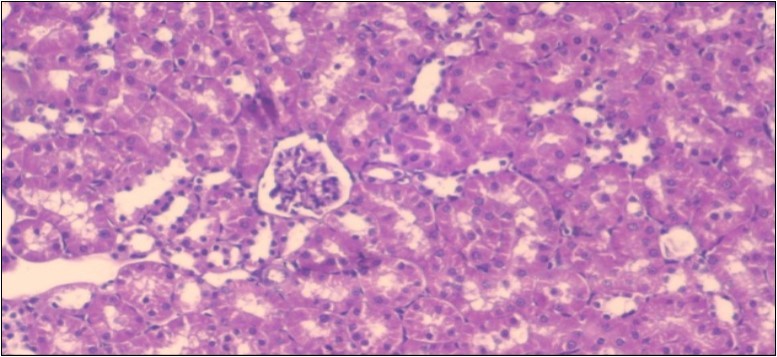
Figure 4.Photomicrogragh of kidney section of treated rat with Ator drug showing improvement in Bowman's capsules with normal glomerular and partial improvement in proximal tubules and distal tubules, (H&E) (40X).
Figure 5.Photomicrograph of kidney section of treated rat with both fennel herb and Ator drug showing highly improved tissue with normal glomerular. Note, most Bowman's capsules and renal tubules, restoring their normal appearance (star). (H&E) (40X).
Effect of Ator, Fennel and Their Combination on the Liver of Obese Rats
The histological examination of the livers of control rats feeding standard diet showed normal architecture hepatocytes, blood sinusoid and central vein, figure 6. The rats which were fed on a (HFD), showed swollen hepatocytes with vacuolated cytoplasm filled with fatty infiltration, congested central vein and disappearance of blood sinusoids, figure 7. By comparison, the liver of rats that were treated by fennel after obesity and control mice observed nearly normal of the hepatocytes with eosinophilic cytoplasm, central vein and clear blood sinusoids and more bi-nucleated cells, figure 8. While examination of rats' liver that were treated by Ator and after the obesity appears, they showed mild fatty change in hepatocytes, few hepatocytes retain is eosinophilic cytoplasm and central vein figure 9. Liver of rats that were treated by fennel and Ator after obesity and control rats are showing nearly normal hepatocytes figure 10.
Figure 6.Photomicrogragh of liver section of control rat showing normal architecture Hepatocytes, blood sinusoid and central vein, (H&E) (40X).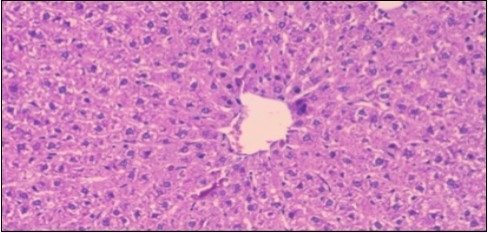
Figure 7.Photomicrograph of liver section of obese rat showing swollen hepatocytes with vacuolated cytoplasm filled with fatty infiltration, congested central vein and disappearance of blood sinusoids, (H&E) (40X).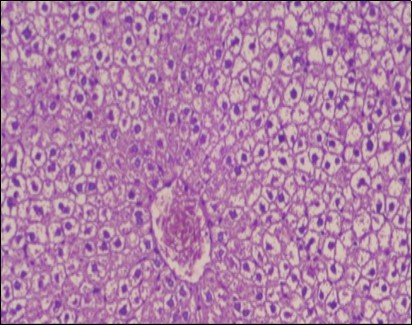
Figure 8.Photomicrograph of liver section of treated rat with fennel herb showing nearly normal of the hepatocytes with eosinophilic cytoplasm, central vein (CV) and clear blood sinusoids and more bi-nucleated cells , (H&E) (40X).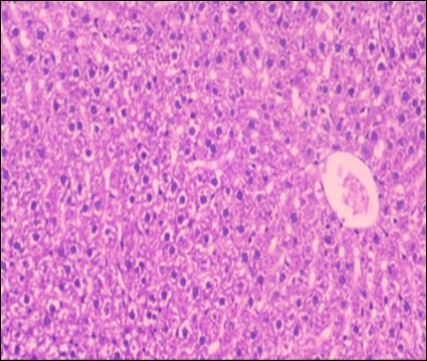
Figure 9.Photomicrogragh of liver section of treated rat with Ator drug showing mild fatty change in hepatocytes, few hepatocytes retain is eosinophilic cytoplasm and central vein , (H&E) (40X).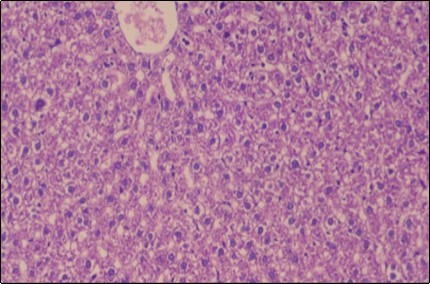
Figure 10.Photomicrograph of liver section of treated rat both with both fennel herb and Ator drug showing nearly normal hepatocytes (H&E) (40X).
Discussion
Obesity is caused by various environmental and genetic factors: one of the main environmental factors causing obesity is the intake of a HFD. In this study, high fat diet (HFD) caused significant increase in the body weight of the obesity animal, this result was in agreement with Galisteo et al.25 observed that food intake in obese rats was significantly increased after 12 or 24 weeks. This means that the total energy intake was higher in obese rats than that in the lean control one. Consumption of high fat diet led to obesity and overweight because it facilitates the development of a positive energy balance leading to an increase in visceral fat deposition, this lead to abdominal obesity in particular 26. Schrauwen-Hinderling et al.27 found that high fat diet feeding is accompanied by molecular adaptation that favors fat storage in muscle rather than oxidation.
In this study the body weight of the group which treated with fennel herb significantly decreases, this results agree with Schone et al.28 reported that food intake in rats treated with either one of the fennel extracts. In this respect, it can be proposed that trypsin inhibitors in fennel reduce food intake and stimulate cholecystokinin release, increasing satiety and that is the reason for fennel's association with weight control. Weight loss Fennel increases the metabolism of fats and sugars in liver and pancreas. It dissolves fat deposits in bloodstream also and allows it to be used as an energy source. These aspects combined with its natural diuretic effect and a reputation as an appetite suppressant makes it an excellent remedy for weight loss 29. In the present study, fennel reduced the body weight gain. This may be due to several isoflavans constituents of fennel as phytoestrogens, which like estradiol, affect the serotonergic system, inhibiting serotonin re-uptake and thereby increasing the levels of serotonin in synaptic clefts, this enhances satiety and promote weight gain 30.
In the current study the rats which treated with atorvastatin significantly decrease the body weight, this result was agreement with Erdmann et al.31 observed that the treatment of over-weight and obesity has been shown to reduce plasma cholesterol. In this study it was observed that combination of fennel and atorvastatin showed significant decrease in the body weight compared to other group and reach to the normal. In this study, high fat diet caused significant increase in the protein of the obese animal. In this study the protein of the group which treated with fennel herb significantly decreases. In current study, the rats which treated with atorvastatin significantly decrease the protein. In this study it was observed that combination of fennel and atorvastatin showed significant decrease in the protein compared to other group and reach to the normal. In the present study the high fat diet caused significant increase in the albumin of the obese animal. In this study the albumin of the group which treated with fennel herb significantly decreases. In current study, the rats which treated with atorvastatin significantly decrease the albumin.
In the present study, HFD caused significant decrease in platelet, this result was agree with Chen et al.32 reported that the effects of naturally-gained obesity on hematological and biochemical parameters are scarce. Chronic inflammation affects many hematological parameters, including erythrocytes, leukocytes and platelets. It is now well-established that obesity results in a state of chronic low-grade inflammation 33. In the current study, the rats which treated with fennel herb showed increase in the platelet count, this result was in disagreement with Kaur 34 who reported that Fennel seeds are also known to have anti-cancerous, antihirsutism, anti-inflammatory, antioxidant, anti-platelet, anti-thrombotic, and anti-spasmodic properties. Previous studies have shown that Fennel seeds contain high levels of nitrites and nitrates. Nitrites and Nitrates are known to play crucial roles in maintaining vascular and digestive functions.
The increased serum LDL level in obese rats has been also recorded in high fat diet supplemented rats 35. This was explained by the decreased HDL level, as recorded in our study, thus decreasing the reverse cholesterol transport from the blood stream to the liver 36. Different fennel extracts seeds used in the present study could reduce serum cholesterol level in obese rats. Non appreciable reduction in serum triglycerides level was recorded in obese rats treated with either one of fennel extracts seeds. In current study, the rats which treated with fennel significant decrease in serum cholesterol and LDL levels accompanied with insignificant decrease in serum triglycerides level. While, significant increase in serum HDL level, this result was agree with Choi and Hwang 6 who observed that fennel methanolic extract seeds treatment could significantly decrease serum LDL level and increase serum HDL level in obese rats. The other fennel extracts could insignificantly reduce LDL level and rise HDL level in obese rats. These results indicated that the constitution of fennel seeds plays an important role in improving blood lipid profile. This could be explained as fennel methanolic extract could significantly increase HDL level. This type of lipoprotein could stimulate the reverse cholesterol transport from the blood stream to the liver 36. Furthermore, it has been shown that fennel, involved in herbal formulation, could delay upper gastrointestinal transit which promotes a decrease in fat and sugar absorption 37.
In the present study treatment with atorvastatin significant decrease in serum cholesterol and LDL levels accompanied with insignificant decrease in serum triglycerides level. While, significant increase in serum HDL level In this study we observed that combination of fennel and atorvastatin showed more effecting compared to other group and reach to the normal. In the current study indicates that the HFD increase kidney funcation (blood urea, creatinine and uric acid), this results was in agreement with Abrass 38 who reported that HF diet groups were associated with high serum cholesterol and triacylglycerol, in addition to histopathological alterations in kidneys. The role of HF as a prominent risk factor for renal disease progression has been increasingly recognized and deterioration of renal function also promote alterations in lipid metabolism. Lipid abnormalities are frequently associated with renal disease. Obese people are at increased risk for proteinuria and chronic kidney disease, independent of concurrent diabetes mellitus or hypertension.
Griffin et al. 39 found that patients with obesity-related glomerulopathy (ORG), defined as glomerular disease in obese patients in the absence of other renal disease-associated conditions, develop proteinuria prior to the onset of azotemia 40. In the current study indicates that fennel significant decrease creatinine and uric acid. The present results were in agreement with Fasset et al.41 who observed that atorvastatin 10 mg would significantly slow the rate of decline of kidney function with chronic kidney disease. In the present study , the obesity rats indicates the increase in the liver function this results was agree with Choi 42 who reported that elevated serum hepatic enzyme activities may be associated with a high prevalence of fatty liver, which is frequently observed in obese humans. In the present study, the rats which treated with fennel herb indicates significant decrease in the liver function this results was in agreement with Ozbek et al.43 who observed that herbal drugs and essential oils of fennel have hepato-protective effects. In the present study, the rats which treated with atorvastatin indicates significant decrease in the liver function this results was agree with Ali et al.44 who showed that oral atorvastatin is capable of improving liver function enzymes (ALT, AST, and ALP) and cause a significant decrement in their serum levels after induction of hypercholesterolemia in rats 45, 46, 47, 48, 49. Previous studies illustrated that LPO and oxidative stress can severely elevated in patients with diabetes and hyperlipidemia 50 and that atorvastatin can improve serum levels of liver enzymes by its antioxidant effect against the liver damage.
The current study indicates that the HFD increase in oxidative stress, these results are in agreement with Diniz et al.50 who reported that oxidative stress was found to be associated with obesity. There is a growing awareness that obesity is a prime risk factor for the development of dyslipidemic profile and that oxidative stress may play a role in various adverse effects of obesity. The present data revealed that the lipid peroxidation product (MDA) recorded significant elevation in obese rats when compared with the lean control one. In the present study, the rats which treated with fennel herb indicate significant decrease in the oxidative stress, this result agreed with Choi and Hwang 6 who reported that the significant reduction in serum MDA level observed in obese rats treated with either one of the fennel extracts could be attributed to the anti-lipid per oxidative capacity of fennel constituents in its methanolic extract. Augmentation of the antioxidant defense system and the anti-lipid per oxidative activity of aqueous fennel extract has been reported 51.
The anti-inflammatory and antioxidant activities of fennel have been reported by Choi and Hwang 6. Anti-oxidant Fennel contains flavonoid anti-oxidants compounds like kaempferol and quercetin which helps eliminate harmful free radicals from the body, thus protecting from infection and aging. Fennel seeds are generally eaten for the taste but also very healthy owing to the nutrition value attached to it. Fennel is used for various health benefits that are derived from its anti-oxidants. In the present study, the rats which treated with atorvastatin indicates significant decrease in the oxidative stress, this results was agree with Koter et al.52 who reported that atorvastatin showed a significant reduction in the MDA level. Medicinal plants can be used in the treatment of various diseases 53, Fennel seeds contain antioxidants as kaemoferol and quercetin that prevent degenerative reactions 54.
Many researchers reported that micro vesicularsteatosis occurred due to changes in enzyme levels of hepatocytes. They emphasized that it was caused by oxidative stress resulting in mitochondrial degenerations 55. The present results were confirmed with histological changes of feeding rats with high fat diet only, which showed vaculation of tunica media and narrowing in the lumen of aorta sections as well as congestion of cardiac blood vessel and hyalinosis of its wall. This result was confirmed by Szilvassy et al.56 who indicated that although hyperlipidemia increases oxidative stress in the cardiovascular system, it renders the heart and the vasculature more susceptible to stress. Ouwens et al. 57 identified that development of hyper-cholestremia which is one of the risk factors for cardio vascular diseases.
Conclusion
The high fat diet administration was associated with a highly significant increase in urea level as compared to the control group. Treatment with fennel, ator and fennel with ator following high fat diet administration exhibited a high drop in this elevation as compared to the obesity group and this indicated also that treatment with fennel with ator was near to the control value.On the other hand, fennel group and ator group supplement successes partially in improving the creatinine level in comparing with the obesity group. And although fennel and ator group indicated significant more decrease in creatinine level as compared to group(3) and group(4) but increase than the control. Uric acid level showed a significant increase in obesity group as compared as to the control value. The administration of fennel, ator and fennel with ator resulted in a partial improvement in the uric acid level as compared to the diabetic group .In the opposite direction the uric acid level of group (5) showed significant decrease comparing to group (3) and group(4). The data reported in the present work indicates that administration of high fat diet provoked a significant increase in serum ALT, AST and ALP. Treatment with (fennel) and (ator) induced a partial recovery but treatment by fennel with ator more improving the ALT, AST and ALP.The finding of this study indicates that the concentration of Malondialdehyde (MDA) and Myeloperoxidase (MPO) in liver homogenates of the fennel and ator group significant decrease than group (3) and group (4) and obesity group. There is a growing awareness that obesity is a prime risk factor for the development of dyslipidemic profile and that oxidative stress may play a role in various adverse effects of obesity.
References
- 1.Abumrad N A, Klein S. (2010) Update on the pathophysiology of obesity. Curr Opin Clin Nutr Metab Care.13: 357-8.
- 2.Brunt E. (2010) Pathology of nonalcoholic fatty liver disease. , Nat. Rev. Gastroenterol Hepatol 7, 195-203.
- 3.Barnes D, Yaffe K. (2011) The projected effect of risk factor reduction on Alzheimer’s disease prevalence. , Lancet Neurol 10, 819-828.
- 4.Saravana Kumar A, Mazumder Avijit, Saravanan V S. (2008) Antihyperlipidemic activity ofCamelliasinensisleaves in Triton WR-1339 induced albino rats. Pharmacognosy magazine. 4(13), 70-64.
- 5.Miller M, Cannon C P, Murphy S A, Qin J, Ray K K et al. (2008) PROVE IT-TIMI 22 Investigators Impact of triglyceride levels beyond low-density lipoprotein cholesterol after acute coronary syndrome in the PROVE IT-TIMI 22 trial. , J Am Coll Cardiol 51, 724-730.
- 6.Choi E M, Hwang J K. (2004) Anti-inflammatory, analgesic and antioxidant activities of the fruit ofFoeniculum vulgare. , Fitoterapia 75, 557-565.
- 7.Kim J H, Hahm D H, Yang D C, Kim J H, Lee H J et al. (2005) Effect of crude saponin of Korean red ginseng on high fat diet induced obesity in the rat.J. , Pharmacol Sci 97, 124-131.
- 8.Amr A, Rezq. (2005) The department of nutrition and food science Faculty of home economics. , Helwan University. Med. J. Cairo Univ 80(2), 101-113.
- 9.Schone F, Vetter A, Hartung H, Bergmann H.A Biertumpfelet al.(2006). Effects of essential oils from fennel (Foeniculiaetheroleum) and caraway (Carviaetheroleum) in pigs. , J. Anim. Physiol. Anim. Nutr 90, 500-510.
- 10.Gornall A J, Bardawill C J, David M M. (1949) Determination of serum proteins by means of the biuret reaction. , J. Biol. Chem 177, 751-66.
- 11.Doumas B T, Waston W A, Biggs H G. (1971) Albumin standard and the measurement of serum albumin with bromocresol green. , Clin. Chim. Acta 31-87.
- 12.Allain C C, Poon L S, Chan R W.Enzymatic determination of total serum cholesterol:. , Clin. Chem 20, 470-5.
- 13.Fassati P, Prencipe I. (1982) Serum triglyceride determined colorimetrically with an enzyme that produces hydrogen peroxide. , Clin. Chem 28, 2077-80.
- 15.Fawcett J K, Soctt J E. (1960) A new simple semimicron method for the determination of urea. , J, Clin. Pathol 13, 156-9.
- 16.Bartles H, Bohmer M, Heirli C. (1972) Serum creatinine determination without protein precipitation. , Clin. Chem. Acta 37.
- 18.DGKC. (1972) Empfehlungen der Deutschen Gesellschaft fur Klinische Chemie. Standard -Method zur Bestimmung der Aktivitat der alkalischen phosphatase. , Z kin Chem u Klin Biochem 10, 191.
- 19.Ohkawa H, Ohishi W, Yagi K. (1979) Assay for lipid peroxides in animal tissue by thiobarbituric acid reaction. , Anal, Biochem 95(2), 351-8.
- 20.Zhang R,et al.(2001) Association between myeloperoxidase levels and risk of coronary artery disease. Jama286.17: 2136-2142.
- 22.Nishikimi M, Roa N A, Yogi K. (1972) The occurrence of superoxide anion in the reaction of reduced phenazine methosulfate and molecular oxygen. , Biochem. Bioph. Res. Common 46(2), 849-54.
- 23.Paglia D E, Valentine W N. (1967) Studies on the qualitative characterization of erythrocyte glutathione peroxidase. , J. Lab. Clin. Med 70, 158-69.
- 24.Drury R A, Wallington E A. (1980) . Carleton’s Histological Techniques. 5thEd., OxfordUniv.Press,NY. 195 .
- 25.Galisteo M, Sanchez M, Vera R, Gonzalez M, Anguera A et al. (2005) A diet supplemented with husks of Plantago ovata reduces the development of endothelial dysfunction, hypertension, and obesity by affecting adiponectin and TNF-α in obese Zucker rats. , J. Nutr 135, 2399-2404.
- 26.Amin K A, Nagy M A. (2009) Effect of creantinine and herbal mixture extract on obesity induced by high fat diet in rats. , Diabetic and Metabolic Syndrome J 1, 1-17.
- 27.Schrauwen-Hinderling V B, Kooi M E, Hesselink M K, Moonen-Kornips E, Schaart G et al. (2005) Intramyocellular lipid content molecular adaptations in response to a 1-week high-fat diet. Obes Res.13:. 2088-94.
- 28.Schone F, Vetter A, Hartung H, Bergmann H, Biertumpfel A. (2006) Effects of essential oils from fennel (Foeniculiaetheroleum) and caraway (Carviaetheroleum) in pigs. , J. Anim. Physiol. Anim. Nutr 90, 500-510.
- 29.Garg C. (2011) (Effect ofFoeniculum vulgareMill. fruits in obesity and associated cardiovascular disorders demonstrated in high fat diet fed albino rats). , J Pharma Biomed Sci 8(19), 1-5.
- 30.Ofir R, Tamir S, Khatib S, Vaya J. (2003) Inhibition of serotonin reuptake by fennel constituents. , J.Mol. Neurosci 20, 135-140.
- 31.R Töpsch Erdmann, Lippl F, Gussmann P, Schusdziarra V.Postprandial response of plasma ghrelin levels to various test meals in relation to food intake, plasma insulin, and glucose.The. , Journal of Clinical Endocrinology and Metabolism 89, 3048-3054.
- 32.Chen Y F, Zm W U, Xie C, Bal S, Zhao L D. (2013) Expression level of il-6 secreted by bone marrow stromal cells in mice with aplastic anemia. , ISRN Hematol.articleID: 986219, 6.
- 33.Solinas G, Karian M. (2010) JNK1 and IKK: molecular links between obesity and metabolic dysfunction. , FASEB J 24, 2596-2611.
- 34.Kaur G J, Arora D S. (2010) Bioactive potential of Anethumgraveolens, Foeniculum vulgare and Trachyspermumammi belonging to the family Umbelliferae-Current status.
- 35.Novelli E L, Diniz Y S, Galhardi C M, Ebaid G M, Rodrigues H G. (2007) Anthropometrical parameters and markers of obesity in rats. , Lab Anim 41, 111-119.
- 36.Raveh O, Pinchuk I, Fainaru M, Lichtenberg D. (2001) Kinetics of lipid peroxidation in mixture of HDL and LDL, mutual effects. Free Radic. , Biol. Med 31, 1486-1497.
- 37.Capasso R, Savino F, Capasso F. (2007) Effects of the herbal formulation ColiMil on upper gastrointestinal transit in mice In vivo. , Phytother. Res 21, 999-1101.
- 38.Abrass C K. (2004) Cellular lipid metabolism and the role of lipids in progressive renal disease. , Am J Nephrol 24, 46-53.
- 39.Griffin K A, Kramer H, Bidani A K. (2008) Adverse renal consequences of obesity. , Am J Physiol Renal Physiol.Apr 294(4), 685-696.
- 40.Kambham N, Markowitz G S, Valeri A M, Lin J, D’Agati V D. (2001) Obesity-related glomerulopathy: An emerging epidemic. , Kidney Int 59, 1498-1509.
- 41.FASSETT R, VENUTHURUPALLI S, GOBE G, COO-MBES J, COOPER M.and HOY W(2008). Biomarkers in chronic kidney disease: A review. , Kidney Int 80, 806-21.
- 42.Choi J W. (2003) Association between elevated serum hepatic enzyme activity and total body fain obese humans. , Ann. Clin. Lab. Sci 33, 257-264.
- 43.Ozbek H, Uğraş S, Dülger H, Bayram I, Tuncer I et al.Oztürk A(2003). Hepatoprotective effect ofFoeniculum vulgareessential oil. , Fitoterapia 74, 317-319.
- 44.Ali S O, Darwish HAEM, Ismail NAEF. (2014) Modulatory effects of curcumin, silybin-phytosome and alpha-R-lipoic acid against thioacetamide-induced liver cirrhosis in rats. , Chemico-Biol. Interact 216, 26-33.
- 45.Radwan E H. (2016) Determination of total hydrocarbon and its relation to amino acids found in two bivalve edible species from Alexandria and El Ismailia coast. , Egypt. J Advances in biology. Vol 9, No 5, 1834-1844.
- 46.Radwan E H. (2009) Impact of Marine Pollution on BivalvePinctada radiata(Leach 1814). PhD Thesis. Faculty of Sci. Univ. of Alex. Radwan EH, Abdel Wahab WM, Radwan KhH(2012).Ecological and physiological studies onPinctada radiata(Leach, 1814) collected from Alexandria coastal water (Mediterranean sea. , Egypt. Egypt J Exp Biol (Zool) 8(2), 223-231.
- 47.Radwan E H, Fahmy G H, MKh Saber. (2017) The impact of some organic and inorganic pollutants on fresh water (Rashid branch, River Nile), Egypt. J of advanced in biology. 10(2), 2133-2145.
- 48.Radwan E H, Hassan A A, Fahmy G H, El Shewemi SS, Sh Salam. (2018) Impact of environmental pollutants and parasites on the ultrastructure of the Nile bolti,Oreochromisauruis. , J of Biosciences and applied Research 4(1), 58-83.
- 49.Kelly G E, Husband A J. (2003) Flavonoidcompounds in the Preventionof prostate cancer. , Methods Mol Med 81, 377-394.
- 50.Diniz Y S, KKHR Rocha, Souza G A, ELB Novelli, Galhardi C M.Ebaid GMX,et al.(2006). Effects of N-acetylcysteine on sucrose-rich diet-induced hyperglycaemia, dyslipidemia and oxidative stress in rats (Report).doi: 10.1016/j.ejphar.2006.05.03. , Eur J Pharmacol 543(13), 151.
- 51.Birdane F M, Cemek M, Birdane Y O, Gulcin I, Buyukokuroglu M E. (2007) Beneficial effects ofFoeniculum vulgareon ethanol-induced acute gastric mucosal injury in rats. , World J. Gastroenterol 13, 607-611.
- 52.Koter M, Broncel M, Chojnowsk-Jezierska J, Klikcznska K, Franiak I. (2002) The effect of atorvastatin on erythrocyte membranes and serum lipids in patients with type-2-hypercholesterolemia. , Eur. J. Clin. Pharmacol 58(8), 501-506.
- 53.Kooti W, Hasanzadeh-Noohi Z, Sharafi-Ahvazi N, Asadi-Samani M, Ashtary-Larky D. (2016) Phytochemistry, pharmacology, and therapeutic uses of black seed (Nigella sativa). , Chinese J. of Natural Medicines 14(10), 732-45.
- 54.Alexandrovich I, Rakovitskaya O, Kolmo E, Sidorova T, Shushunov S. (2003) The effect of fennel (Foeniculum vulgare) seed oil emulsion in infantile colic: a randomized, placebo-controlled study. , Altern Ther Health Med 9, 58-61.
- 55.Natarajan S K, Eapen C E, Pullimood A B, Balasubramanian K A. (2006) Oxidative stress in experimental liver microvesicu - larsteatosis: role of mitochondria and peroxisomes. , J Gas-troenterolHepatol; 2, 1240-9.
Cited by (7)
- 1.Abdoon Ahmed Sabry S., Hegazy Amany M., Abdel-Azeem Amal S., Al-Atrash Ahmed M., Mohammed Dina Mostafa, 2024, The protective effects of some herbs on mitigating HFD-induced obesity via enhancing biochemical indicators and fertility in female rats, Heliyon, 10(9), e30249, 10.1016/j.heliyon.2024.e30249
- 2.Khan Riyaz, Ahmad Wajid, Pathan Razia, Jain Vishal, Rajput Dipali, 2024, Fennel Seed Oil's Protective effect on Serum Potassium and Sodium Ions from Formaldehyde-Induced Toxicity in Wistar Rats, Research Journal of Pharmaceutical Dosage Forms and Technology, (), 205, 10.52711/0975-4377.2024.00032
- 3.Ghaffar Bareera, Zafar Umrah, Alvi Samara Qaiser, Fatima Arooj, Amjad Umar Ali, et al, 2025, Therapeutic Effect of Fennel Seeds in the Management of Obesity, Pakistan BioMedical Journal, (), 08, 10.54393/pbmj.v8i2.1217
- 4.Noreen Sana, Rehman Habib‐ur, Tufail Tabussam, Badar Ul Ain Huma, Awuchi Chinaza Godswill, 2023, Secoisolariciresinol diglucoside and anethole ameliorate lipid abnormalities, oxidative injury, hypercholesterolemia, heart, and liver conditions, Food Science & Nutrition, 11(6), 2620, 10.1002/fsn3.3250
- 5.Barakat A I, Radwan EH, De Patricio, 2020, Effect of Trigonella Foenum against Ethylene Diamine Tetra Acetic Acid induced Nephrotoxicity in Male Albino Rats, Journal of Zoological Research, 1(1), 32, 10.14302/issn.2694-2275.jzr-20-3435
- 6.Noreen Sana, Tufail Tabussam, Tufail Tanazzam, Bader Ul Ain Huma, Jaffar Hafiza Madiha, et al, 2023, Cookies enriched with anethole and secoisolariciresinol diglucoside from flaxseed and fennel seeds improve hypercholesterolemia, lipid profile, and liver functions: A pilot study, Food Science & Nutrition, 11(7), 4211, 10.1002/fsn3.3433
- 7.Jaiswal Aishwarya, Pathania Vanshdeep, Lakshmi A Jyothi, 2021, An exploratory trial of food formulations with enhanced bioaccessibility of iron and zinc aided by spices, LWT, 143(), 111122, 10.1016/j.lwt.2021.111122
