Embolization for Perimedullary Arteriovenous Fistulae: Pioneering Experience in Peru
Abstract
Background
Perimedullary arteriovenous fistulae, or type IV spinal cord arteriovenous malformations, are very rare and not well-known lesions. This paper aims to present our endovascular experience with these lesions.
Methods
We report our experience with 4 patients with perimedullary arteriovenous fistulae, subtypes b and c (macrofistulae), exhibiting severe neurological impairment. The patients were treated with endovascular embolization.
Results
Complete fistula eradication was achieved in all of them. One complication occurred. We discuss the natural history, pathophysiology, clinical presentation, prognosis and embolization techniques, along with the angiographic and clinical outcomes.
Conclusion
Our experience with endovascular embolization as an upfront treatment allowed us to eradicate these lesions in a safe and effective way, arresting the clinical worsening and reversing partially or completely the neurological injury in most of our cases.
Author Contributions
Academic Editor: Sasho Stoleski, Institute of Occupational Health of R. Macedonia, WHO CC and Ga2len CC, Macedonia.
Checked for plagiarism: Yes
Review by: Single-blind
Copyright © 2021 Andrés R. Plasencia
 This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Competing interests
The authors have declared that no competing interests exist.
Citation:
Introduction
First described by Djindjian et al, 7 perimedullary arteriovenous fistulae (PMAVFs) are direct pial arteriovenous shunts between an artery and a vein without an intervening nidus, lying alone the surface of the spinal cord or the proximal filum terminale. Their blood supply comes from the anterior spinal artery (ASA), posterior spinal artery (PSA), or both, and the venous drainage is through the perimedullary veins. These lesions account for approximately 15 to 40% of all spinal cord vascular arteriovenous malformations (SCAVMs).
PMAFVs were later subclassified by Merland in three subtypes according to their size: Type I, II and III, corresponding to small, large and giant subtypes, respectively. 7, 19, 31 Type I PMAVF is a single and thin AVF fed by the ASA and located in the anterior surface of the conus medullaris or the filum terminale associated with a mildly dilated ascending draining vein. This type of lesion is hemodynamically analogous to a spinal dural arteriovenous fistula (SDAVF). Type II PMAVF has an intermediate size and increased blood flow. It is supplied by arterial feeders, one arising from the ASA and from one or two PSAs. Its fistulous site usually lays either in the anterior or posterolateral surface of the conus medullaris, draining into an ascending enlarged and convoluted perimedullary venous network. Type III PMAVF is a single and gigantic fistulae located in the cervical or thoracic spinal cord with a high-flow shunt supplied by multiple branches arising from ASA or PSA, draining into a large and tortuous venous network exiting into segmental metameric veins. Anson and Spetzler, 2 separated Type IV PMAVFs into IVa, IVb and IVc. Additionally, in a revised classification, Rodesch et al, 27 separated these lesions into micro (type a) and macrofistulae (types b and c). This new classification has a pragmatic relevance when it comes to selecting the proper treatment modality since, due to its small size and flow, type a PMAVFs (microfistulae) are usually treated surgically, while types b and c (macrofistulae) are more amenable to embolization.
In this report, we described retrospectively our experience in the management of Type II and III PMAVFs, which correspond to type b and c (macrofistulae), and discuss the natural history, pathophysiology, clinical presentation, angiographic and clinical outcomes, prognosis and evolving embolization techniques for the treatment of these complex lesions.
Materials and Methods
Patient Population
We report retrospectively 4 consecutive cases of PMAVFs treated by the senior authors (ARP and FGS) between January 1996 and January 2020 with endovascular embolization as an upfront therapeutic modality at our institutions. There were 3 females and 1 male with ages ranging between 6 months and 31 years (mean 14.3 years old). Their clinical presentation started with a slow and progressive limb sensorimotor deficit associated with bladder and bowel dysfunction. None of the cases showed evidence of subarachnoid hemorrhage or intramedullary bleeding. The time interval between the beginning of the symptoms and the treatment ranged between 6 and 24 months (mean 7.5 months). The clinical findings were based on the Aminoff-Logue Scale (ALS). 1 Our patients’ pre and postoperative neurological status, as well as the angiographic postembolization results, are summarized in Table 1. Demonstrative images with procedural descriptions are showed in Figure 1, Figure 2, Figure 3, Figure 4 corresponding to cases 1-4 in chronological order. The collected literature on PMAVF was obtained from Pubmed since 1974 and an extensive review of the literature was performed.
Figure 1.Case # 1: a) T2W sagittal and b) coronal MRI images showing a large bilobed varix in the anterior aspect of the spinal cord at the vertebral levels of C7 and T1 (star). Notice the large venous drainage ascending ventrally from the cervical spinal cord towards the brainstem (thin arrow). A previously C3 to C7 “decompressive” laminectomy was performed (arrows). c) Right thyrocervical trunk demonstrates that the main feeder to the fistula is the right artery of the cervical cord enlargement (short arrow). d) Another feeder from the ASA, branch of the left vertebral artery is visualized (thin arrow). The venous varix is shown (star). e) Roadmap image at the time of micro catheterization of the left ASA. The tip of the microcatheter (arrowhead) was further advanced to the fistulous site (open arrow) and 0.8 cc of 90% n-bca in lipiodol was injected. Also notice the glue cast injected in the artery of the cervical enlargement (thin arrow). f) MRI T2W FLAIR coronal and g) sagittal (postoperative day 5) showing fresh clot in the venous varix (star) with retrograde thrombosis of the ascending draining veins (arrows). The MR signal of the spinal cord remained normal.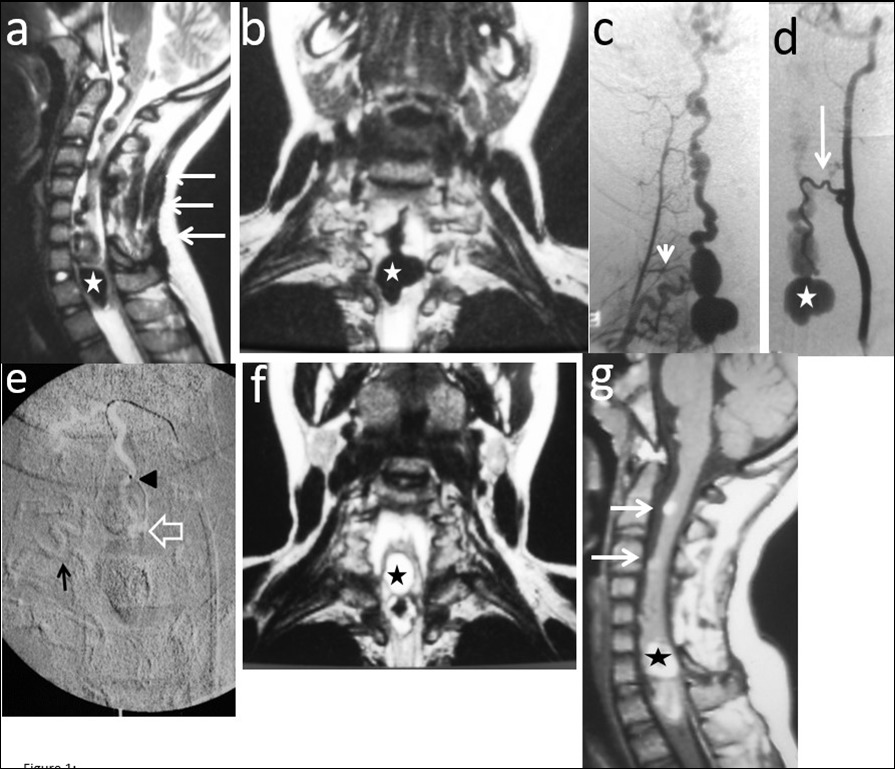
Figure 2.Case # 2: a) Coronal and b) sagittal T2W MRI shows large venous varix compressing ventrally the cervical cord at C5-C6 levels causing moderate central hyperintensity above, and mild below the cervical cord. Ascending convoluting draining veins reach the brainstem. c) d) and e) Angiograms showing right and left arteries of the cervical enlargement as well as a PSA of the right vertebral artery. All of them converge and shunt the varix at the same fistulous point. The ASA branches off the left artery of the cervical enlargement (thin arrows). f) DSA shows the right PSA feeder catheterized proximally. An injection of 30% n-bca in lipiodol was performed reaching the fistulous point (arrow). A thin layer of glue cast is seen in the lateral wall of the ascending vein (arrowheads) g) and h) Post-embolization right ascending cervical artery angiogram showing an arterial blockade at the entry of the fistulous point (star) preserving its anastomosis with the contralateral artery (open arrow). Notice that by reflux, the left artery of the cervical enlargement fills the ASA (thin arrows). Four hours later, the patient suddenly developed quadriplegia probably due to venous and/or ASA thrombosis. Patient lost to follow-up after 2 months.
Figure 3.Case # 3: a) T2W MR sagittal and b) axial non contrast spinal MRI, showing flow voids in the dorsal aspect of conus and epiconus (open arrows) with hypersignal extending towards the lower dorsal spinal cord (arrowhead in a). c and d) Segmental left T11 angiogram in AP and lateral projections. The artery branches off to an enlarged ASA (Artery of Adamkiewicz) and also a thinner PSA feeding the PMAVF. The fistula is located posterolaterally (thin arrow in c) and d), draining downwards (arrowhead). e) Injection of 33% n-BCA with rapid microcatheter removal. See the tip of the microcatheter in the curve of the PSA (open arrow). The glue reached the fistulous site and the draining vein (open arrowheads) f) Left T11 angiogram following embolization did not show any shunt and only remnants of the abnormal draining veins were seen (star) with preservation of the ASA axis (thin arrows). g) An angiogram performed 3 months postembolization demonstrated complete eradication of the AVF and preservation of the artery of Adamkiewicz (thin arrows).
Figure 4.Case 4: a) 3D CTA shows a gigantic venous pseudoaneurysm occupying the spinal canal at the T12-L1 vertebral levels with tortuous veins above and below the lesion. b) and c) 2D reformatted images. d) and e) Selective right L3 angiogram through a 4F guiding catheter (arrow) in lateral and AP projections showing an enlarged PSA feeding a huge venous varix with ascending and descending perimedullary veins (thin arrows) and with segmental epidural draining veins at the fistula level (arrowhead).f) A microcatheter was navigated up to pseudoaneurysm (arrow) g) In the venous sac, 21 GDC coils were deployed (open arrow) and the microcatheter tip was pulled back to the fistulous site followed by Onyx 18 injection to fill up the foot of the ascending draining vein (thin arrows). h) Post-embolization abdominal aortogram (late phase) failed to demonstrate any remaining shunt. i) to l) Multiplanar T2 MR images 6 months’ post-embolization showing absence of flow, and artifacts due to coils and Onyx (arrows). No spinal cord hyperintensity was observed. Patient starting to walk independently with minimal right foot dorsiflexion paresis. He gained complete sphincter control.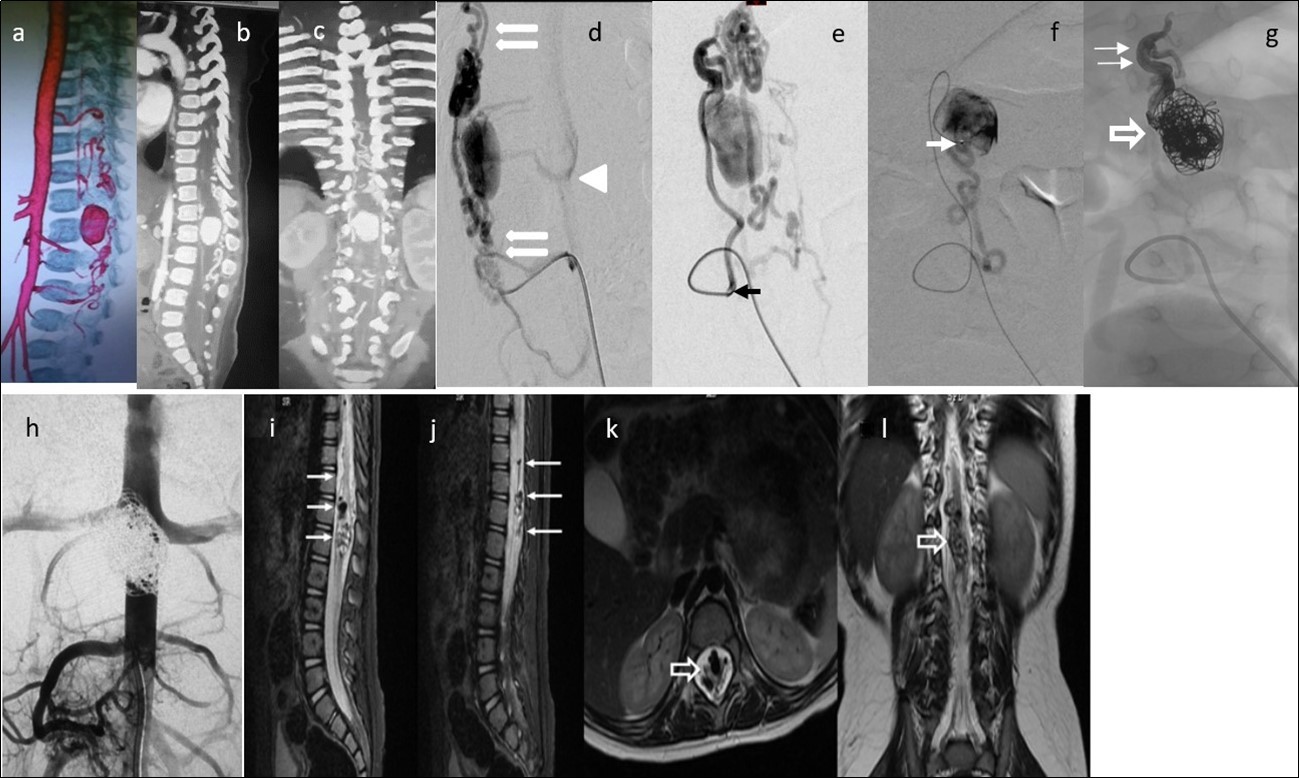
Neuroimaging and Angiographic Evaluation
The pre and post-embolization CT, MRI and angiographic images available are described in Table 2. A selective spinal angiography was performed with transarterial femoral access. 5F Cobra catheters and 0.035” hydrophilic guidewires were utilized in all cases. Non-ionic iso-osmolar contrast material was manually injected. A complete axis spinal angiogram was obtained. Digital subtraction angiography (DSA) images in anteroposterior and lateral projections were obtained at a rate between 4 to 7.5 frames per second. The location, anatomy and hemodynamics of the lesions were studied with superselective injections to evaluate the angioarchitecture of the feeders, venous varix, and draining veins as well as the flow rate in order to select the optimal route to the fistulous site and the embolization material.
Table 1. PMAVFs’ clinical and neuroimaging presentation and outcome| Case N° | Age/Sex | Clinical features/ALS | Fistula site | Fistulasubtype | EmbolicAgent | FistulaOblite-ration | ClinicalCourse/Follow-up period (months) |
| 1 | 12y/F | QP+BBD/5 | C7-T1 | C | n-bca | C | CompleteRecovery/ 6 m |
| 2 | 14y/F | Qp+Tinnitus+BBD/4 | C4-C6 | C | n-bca | C | Delayed worsening QP/2 m |
| 3 | 3y/M | Pp+BBD/2 | T10-T11 | B | n-bca | C | SignificantImprovement/8 m |
| 4 | 6m/F | PP+BBD/5 | T11-T12 | C | Coils &Onyx 18 | C | Complete recovery/12 m |
| Case N° | CT/ 3D CTA | MRI | Angiography: Arterial feeders | Angiography: Venous phase |
| 1 | C3-C6 decompressive laminectomy | Bilobed LVP ventrally and compressing the cervical cord @ C7-T1 vertebral level | ASA from Lt. vertebral artery and 2 larger arteries of the cervical enlargement | Dilated ascending tortuous veins ventral to the cord and draining to a large vein ventral to the brainstem |
| 2 | NA | Ovoid GVP ventrally and compressing the spinal cord @ C5-C6 vertebral level. Spinal cord narrowing and T2WI hyperintensity | ASA from Lt vertebral artery and 2 larger arteries of the cervical enlargement | Dilated ascending tortuous veins ventral to the cord and draining to a large vein ventral to the brainstem |
| 3 | LVP@T12 vertebral level w/many exiting veins rostral and caudally | LVP @ T12 vertebral level, posterolaterally to the conus/epiconus w/T2WI hiperintensity | Single feeder: PSA branching from a radiculomedullary artery arising from Lt. T11 segmental artery | Dilated and convoluted descending venous drainage towards foraminal veins @ Rt. L1-L2 |
| 4 | GVP@T11-12 vertebral levels ventrally and compressing the spinal cord | NA | 2 main feeders:Rt. T12 & Rt. L3 which shunted at the same fistulous point. Rt. L3 was more accessible and chosen for embolization | Convoluted ascending venous drainage in the posterior surface of the conus and epiconus |
| Fistula subtype | A | B | C |
| Size | Small | Intermediate | Giant |
| Feeders | Single shunt, usually a terminal branch of a thin ASA, mainly the A. of Adamkiewicz | Few shunts, rarely single, supplied by one ASA and one or more PSA | One or multiple large feeders converging into a single large fistula. One ASA usually dominant |
| Location | Ventral to the conus or to the filum terminale | Posterolaterally against the conus medullaris | Ventral to the cervical or thoracic spinal cord |
| Blood Flow | Relatively Slow | More rapid than type a | Markedly increased |
| Venous drainage | Slightly dilated and tortuous ascending perimedullary veins | Greater dilatation (ampullary) immediately after the fistulous site with slow ascending venous drainage recruiting the perimedullary plexus | Severely dilated venous drainage with ectasia and venous aneurysms near or distal to the shunt with local venous drainage to the epidural plexus |
Endovascular Intervention
After signed consent and under general intravenous anesthesia, a 5F femoral sheath was placed in the femoral artery. Then, coaxially, with a Cobra I or II 5F catheter assembled with a rotating hemostatic valve and a hydrophilic guidewire, a complete spinal angiography was done from cervical to sacral segmental arteries with special interest in locating the lesion as well as evaluating the ASA axis, mainly the anterior spinal artery of Adamkiewicz. Once identified, the safest and easiest for embolization feeder of the AVF was catheterized with the Cobra catheter and then exchanged for a 5F guiding catheter. After magnified selective angiography was performed and under roadmap using coaxial technique, a microcatheter (1.5F to 2.3F) over a microguidewire (0.014”, 0.012”, 0.010” and 0.007”) was navigated to reach the fistulous site itself or immediately proximal to it. Any eventual normal arterial collateral detected arising from the arterial feeder was then surpassed to avoid inadvertent occlusion and ischemia. Once in position, the microguidewire was removed and a superselective microangiography was done to evaluate precisely the angioarchitecture and dynamic features of the lesion in order to select the optimal embolic agent and delivery technique. Then, the microcatheter was flushed with a normal saline solution followed by the injection of the embolic materials: n-butyl cyanoacrylate (n-bca), or detachable coils and Onyx-18. Contrast injections through the guiding catheter were performed at different times of the intervention to assess the degree of occlusion and the integrity of the normal vasculature (see Table 2).
Results
Neuroimaging, Angiographic Results
Two out of 4 patients were diagnosed and studied preoperatively with both spinal MRI and CT scans, 1 patient with MRI and the other patient with CT/CT Angiography with 3D reconstruction. Large and tortuous flow voids with large to giant venous pouches or pseudoaneurysms (3 of our cases - type c) causing compression of the spinal cord were found lying ventrally and, in another one, posterolaterally to the spinal cord. Hyperintensity in T2W sequences in the spinal cord was noted in all of them. There were no cases where a hemorrhage was exhibited. The angiographic studies confirmed the 4 large or giant pouches at the draining vein fed by ASA and or PSA and ascending (2 cases), descending (1 case) or metameric (1 case) venous drainage. The blood flow was moderate in 1 case and very fast in the other 3 patients (see Table 2 and Figure 1, Figure 2, Figure 3, Figure 4).
Embolization Results
Transarterial microcatheterization followed by embolization resulted in complete PMAVF obliteration in all of our 4 patients preserving the ASA axis (100%). We used n-bca in 3 patients and Onyx-18 with detachable coils in another one (Table 1). Out of all cases, there was only one angiographic follow-up.
Clinical Results
Table 1 summarizes our patients’ neurological status by means of the Aminoff-Logue scale of motor disability.1 All of them showed rapid neurological improvement from the early hours of the postoperative period to the long term follow-up except by one (patient 2), who, 4 hours after improvement, suddenly developed quadriplegia. This patient was admitted to the ICU for 9 days with mechanical ventilation and, once regained breathe control, began motor rehabilitation, achieving proximal upper limbs movement, and used a wheelchair at 6 months of clinical follow-up, moving from grade 4 to grade 5 of disability. Out of the other three, two type c PMAVF experienced complete remission of their neurological deficits, while another (patient 3, type b PMAVF) improved from grade 2 to grade 1 (see Table 1). Our clinical follow-up period ranged from 2 to 12 months.
Discussion
Classification
PMAVFs were first described by Djindjian in 1977. 7 Later, Heros classified them as type IV SCAVMs. 14 The group of La Riboisiere, leaded by Merland,12, 18, 22 further subclassified them in subtypes I, II and III which Anson and Spetzler later renamed them IVa, IVb and IVc to avoid confusion. 2 Type IV PMAVFs are extramedullary AVFs usually supplied by the ASA and less frequently by the PSA, draining into a large and convoluted venous network. Most of them are seated in the conus medullaris and or cauda equine, and less frequently in the cervical or thoracic spinal cord. 2, 14, Table 3 summarizes the topographical, angioarchitectural and hemodynamical features of the three subtypes of PMAVF. Out of our 4 cases, there were 3 giants (type b) and 1 of intermediate size (type b).
Epidemiology and Clinical Features
Due to its rarity and the few case series and reported cases, the incidence and prevalence of PMAVF remains unknown. 7, 22, 29 The clinical expression of a PMAVF occurs usually between 14 to 42 years and affect both sexes equally. 12 Nevertheless, our case 4 is a 6-month old infant with an IVc fistula which probably represents the youngest case reported in the literature to this day.
The usual symptoms of a PMAVF are intramedullary or subarachnoid bleeding or, less frequently, myelopathy or radiculopathy due to mass effect. 12, 19, 22 All of our patients had progressive myelopathy and none of them showed signs of bleeding.
Etiology and Pathophysiology
The congenital nature of the PMAVF is generally accepted. Macrofistulae with giant venous pouches are commonly encountered in patients with hereditary hemorrhagic telangiectasia. 2, 3, 5, 7, 12, 14, 20, 21, 22 However, few cases of past history of spinal trauma and spinal surgery for tumor removal have also been reported. 3, 12, 17 Interestingly, no cases of hereditary hemorrhagic telangiectasia were present among our 4 cases.
The pathophysiology of the neurological damage to the spinal cord in patients harboring SCAVMs is still controversial. Four mechanisms usually recognized are: hemorrhage (subarachnoid or intramedullary), arterial steal, mass effect, and venous hypertension. In practical terms, probably more than one mechanism plays a role in different proportions in the different types of SCAVMs. 19, 13
It is accepted that spinal cord damage associated with types IV a and b PMAVFs mostly results from extensive venous ischemic disease such as seen in DAVFs, 13, 23, 29, 30 and many authors have reported patients whose clinical disability does not improve despite complete and permanent SDAVF obliteration, suggesting the existence of yet unknown pathophysiological mechanisms. 15, 21 Conversely, radiculomyelopathy seen in type c PMAVF may be essentially related to mass effect and a direct relationship between their obliteration and clinical outcome has been shown, resembling the pathophysiology of intramedullary AVMs. 11, 22 All of our 4 embolized PMAVFs (three type c and one type b) resulted in complete fistula obliteration. Out of them, two made a complete clinical remission of the neurological deficit and another partial significant improvement, while another patient (case 2) unfortunately developed quadriplegia 4 hours post complete embolization, probably related to delayed retrograde ascending venous thrombosis.
Neuroimaging, Angiography and Embolization (See Tables 1 and 2)
MRI in PMAVF patients shows striking vascular lesions. 4, 22, 32 Spinal angiography is mandatory. Selective and superselective studies define the type and subtype of SCAVM and demonstrate its angioarchitecture and hemodynamics in order to design an operative strategy aiming to obliterate the fistula sparing normal arteries by adequate selection of the embolization technique and embolization materials.
PMAVFs may be treated surgically, endovascularly or by a combination of the two. The treatment should be completed as early as diagnosed due to its relentless progressive injury to the spinal cord seen in the natural history. 16, 22, 24, 25
The therapeutic modality differs according to lesion size. In general terms, PMAVFs type a are usually very difficult to microcatheterize due to the relative large extent and very small caliber of the arterial feeder involved and surgery is primarily indicated. 12, 22 However, due to the development of tiny and lubricious last generation microcatheters and microguidewires, this principle has been challenged by Oran et al who achieved complete obliteration of type b PMAVF with good neurological outcomes in 4 out of their 5 cases. 24 Regarding PMAVFs Types b and c (macrofistulae), embolization is a reasonable therapeutic choice due to the good results in the series reported in the literature. 8, 11, 28
We used transarterial approach and a single session achieving complete obliteration of the fistulae while preserving the normal vasculature, mainly the ASA, in all of our 4 cases using either different concentrations of n-bca alone tailored to the blood flow velocity (cases 1,2 and 3), or a combination of coils and onyx 18 (case 4). In this last case, the high flow fistula and the presence of a giant pseudoaneurysm at the venous varix compressing the spinal cord prompted us to deploy coils in the sac and in the draining vein to slow down the blood flow and decrease the chance of distal embolism. To date, we do not have Onyx of higher concentration than Onyx18 available in our country. In high flow macrofistulae, our strategy to slow down the flow to allow casting of the polymerizing embolic mixture in the fistulous site avoiding distal embolism includes lowering mean arterial pressure down to 60-70 mm Hg pharmacologically, by embolizing with concentrations of 66% of n-bca in lipiodol, as well as the placement of detachable coils in the event of very high flow with pseudoaneurysms at or very close to the fistulous site as a first step, to trap the liquid embolic material injected as second step (see Figure 1, Figure 2, Figure 3, Figure 4).
Clinical neurological remission was achieved in 3 of our 4 patients, complete in 2 (cases 1 and 4), and almost complete remission in 1 (case 3). Our case 2 which showed signs of moderate quadriparesis, developed abrupt quadriplegia with respiratory failure 4 hours after a successful embolization. The patient required intubation and mechanical ventilation. An emergency CT scan (not shown) showed fresh clot in the varix and ascending veins with spinal cord edema. We attributed this bad outcome to delayed ascending retrograde venous thrombosis, which is a serious condition associated with severe neurological sequelae and with a high mortality rate. To avoid this complication, peri and postoperative systemic heparinization has been recommended. 6, 9 Among the 4 embolized PMAVFs, only one patient had an angiographic follow-up (case 3). The study was indicated due to non-complete neurological remission achieved at 3 months postoperatively. Persistent fistula obliteration with preservation of the anatomy and flow of the ASA was again demonstrated. This observation agrees with those authors who have hypothesized that the patients without complete neurological improvement, despite complete and permanent PMAVF obliteration, probably have the same nature of diffuse chronic leptomeningeal venous myelopathy, or as yet others unknown mechanisms of non-reversible spinal damage. 15, 21
Conclusion
Our experience with 4 young patients harboring PMAVF of subtypes b and c (macrofistulae) showed a severe and progressive neurological impairment with poor prognosis if left untreated. PMAVFs represent heterogeneous lesions whose angioarchitectural features dictate its optimal treatment. An early diagnosis usually by MRI imaging must prompt to perform a complete spinal angiography. A meticulous and repeated study of the anatomy and the blood flow of these rare lesions are key factors needed for the proper therapeutic strategy and the optimal selection of the endovascular technique and embolic materials. The evolving advances in endovascular technologies and techniques, as well as the wide availability of embolic materials and the improvement of our learning curve, is making endovascular embolization an upfront intervention as shown here, to eradicate within the margins of safety and definitely these fistulae, arresting the clinical worsening and reversing partially or completely the neurological damage caused by these rare and complex lesions.
Acknowledgements
Not a single case of the main author (AP) could have been possible without the teachings and support of Dr. Fernando Viñuela, MD, Emeritus Professor of Interventional Neuroradiology, UCLA Medical Center, USA.
References
- 1.Aminoff M J, Logue V. (1994) The prognosis of patients with spinal vascular malformations. , Brain 97, 211-18.
- 2.Anson J A, Spetzler R F. (1992) Classification of spinal arteriovenous malformations and implications for treatment. , BNI Q 8, 2-8.
- 3.Barrow D L, Colohan A R, Dawson R. (1994) Intradural perimedullary arteriovenous fistulas (type IV spinal cord arteriovenous malformations). , J Neurosurg 81, 221-9.
- 4.Boo S, Hartel J, Hogg J P. (2010) Vascular abnormalities of the spine: an imaging review. Curr Probl Diagn Radiol. 39, 110-7.
- 5.Cho K T, Lee D Y, Chung C K, Han M H, Kim H J. (2005) Treatment of spinal cord arteriovenous fistula: embolization vs surgery. Neurosurgery. 56, 232-9.
- 6.Cogen P, Stein B M. (1983) Spinal cord arteriovenous malformations with significant intramedullary components. , J Neurosurg 59, 471-8.
- 7.Djindjian M, Djindjian R, Rey A, Hurth M, Houdart R. (1977) Intradural extramedullary spinal arterio-venous malformations fed by the anterior spinal artery. Surg Neurol. 8, 85-93.
- 8.Endo T, Endo H, Sato K, Matsumoto Y, Tominaga T. (2016) Surgical and Endovascular treatment of spinal AVMs. Neurol Med Chir (Tokyo). 56, 457-64.
- 9.Gaikwad S B, Mishra N K, Goyal M, Padma M V, Sharma A. (1997) Spinal cord arteriovenous fistula associated with a giant venous pouch in a three-year-old child. Intervent Neuroradiol. 3, 2247-53.
- 10.Gobin Y P, Houdart E, Casasco A, Merland J J. (1993) Endovascular therapy for arteriovenous malformations and fistulae in the spinal cord. Semin Intervent Radiol. 10, 227-42.
- 11.Gross B A, Du R. (2013) Spinal pial (Type IV) arteriovenous fistulae: a systematic pooled analysis of demographics, hemorrhage risk and treatment results. , Neurosurgery.Discussion151 73, 141-51.
- 12.Gueguen B, Merland J J, Riche M C, Rey A. (1987) Vascular malformations of the spinal cord: intrathecal perimedullary arteriovenous perimedullary arteriovenous fistulas fed by medullary arteries. Neurology. 37, 969-79.
- 13.Hassler W, Thron A, Grote E. (1989) Hemodynamics of spinal dural arteriovenous fistulas: An intraoperative study. , J Neurosurg 70, 360-70.
- 14.Heros R C, Debrun G M, Ojemann R G, Lasjaunias P L, Naessens P J. (1986) Direct spinal arteriovenous fistula: a new type of spinal AVM. , J Neurosurg 64, 134-9.
- 15.Kendall B E, Logue V. (1977) Spinal epidural angiomatous malformations draining into intrathecal veins. Neuroradiology. 13, 181-9.
- 16.Lv X, Li Y, Yang X, Jiang C, Wu Z. (2012) Endovascular embolization for symptomatic perimedullary AVF and intramedullary AVM: a series and a literature review. Neuroradiology. 54, 349-59.
- 17.Meng X, Zhang H, Chen Y, Ling F. (2010) Traumatic spinal perimedullary arteriovenous fistula: a case report. , Acta Neurochir (Wien) 152, 1407-10.
- 18.Merland J J. (1987) Reizine D: Treatment of arteriovenous spinal cord malformations Semin Intervent Radiol. 4, 281-90.
- 19.Merland J J, Riche M C, Chiras J. (1980) Les fistules arterioveineuses intracanalaires extramedullaires a drainage veneuse medullaire. , J Neuroradiol 7, 271-320.
- 20.Modic M T, Masaryk T J, Paushter D. (1986) Magnetic resonance imaging of the spine. Radiol Clin North Am. 24, 229-45.
- 21.Mourier K L, Gelbert F, Rey A, Assouline E, George B et al. (1989) Spinal dural arteriovenous malformations with perimedullary drainage. Acta Neurochir (Wien). 100, 136-41.
- 22.Mourier K L, Gobin Y P, George B, Lot G, Merland J J. (1993) Intradural perimedullary arteriovenous fistulae: results of surgical and endovascular treatment in a series of 35 cases. Neurosurgery. 32, 885-91.
- 23.Oldfield E H, Doppman J L. (1986) Spinal Arteriovenous Malformations. Clinical Neurosurgery. , Baltimore, Williams 34, 161-83.
- 24.Oran I, Parildar M, Derbent A. (2005) Treatment of slow flow (type I) perimedullary spinal arteriovenous fistulas with special reference to embolization. , AJNR Am J Neuroradiol 26, 2582-6.
- 25.Pasqualetto L, Papa R, Isalberti M, Nuzzi N P, Branca V. (2011) The endovascular treatment of a spinal perimedullary arteriovenous fistula with coils: a case report. , J Neurointerv Surg 3, 88-91.
- 26.Riche M C, Melki J P, Merland J J. (1983) Embolization of spinal cord vascular malformations via the anterior spinal artery. , Am J Neuroradiol 4, 378-81.
- 27.Rodesch G, Hurth M, Alvarez H, Tadié M, Lasjaunias P. (2002) Classification of spinal cord arteriovenous shunts: Proposal for a reappraisal-The Bicetre experience with 155 consecutive patients treated between 1981 and 1999. Neurosurgery. 51, 374-80.
- 28.Rodesch G, Hurth M, Alvarez H, Tadié M, Lasjaunias P. (1981) Spinal cord intradural arteriovenous fistulae: Anatomic, clinical, and therapeutic considerations in a series of 32 consecutive patients seen between. 57, 973-83.
- 29.Rosenblum B, Oldfield E H, Doppman J L, G Di Chiro. (1987) Spinal arteriovenous malformations: a comparison of dural arteriovenous fistulas and intradural AVM in 81 patients. , J Neurosurg 67, 795-802.
- 30.Symon L, Kuyama H, Kendall B. (1984) Dural arteriovenous malformations of the spine. Clinical features and surgical results in 55 cases. , J Neurosurg 60, 238-47.
- 31.Takai K. (2017) Spinal arteriovenous shunts: Angioarchitecture and historical changes in classification. Neurol Med Chir (Tokio). 57, 356-65.
