Ozone/Oxygen Molecules Exert Mild Oxidative Stress on Testis Mitochondria Isolated from the Rat Testicular Ischemia/Reperfusion Injury
Abstract
Testicular ischemia-reperfusion injury is an urgent situation which needs a timely and precise diagnosis for prevention of testis damages. Here in we investigated ozone/oxygen therapy in Testicular Ischemia/Reperfusion Injury. For this purpose, animals (rats) were divided to four groups; control, torsion/detortion, torsion/detortion + ozone/oxygen (30 µg/ml) and only ozone/oxygen. Four hours after detorsion, in all groups orchiectomy was done -- for measuring the oxidative stress and mitochondrial toxicity parameters. Also, we preformed analysis of testicular spermatogenesis after 90 days. Our data showed that testicular torsion-detorsion induced significant increase in mitochondrial toxicity and decrease of spermatogenesis, malondialdehyde and GSSG levels were shown. Also, spermatogenesis, a remarkable decrease in malondialdehyde GSSG levels and mitochondrial toxicities were observed when compared with torsion-detorsion group. Obtained results for this research showed that ozone/oxygen therapy enhance antioxidant properties in the spermatogenic cells and protects testes from ischemia-reperfusion injury.
Author Contributions
Academic Editor: Mona Hassan, Department of Anatomy and Embryology, Faculty of Medicine, Suez Canal University, Ismailia, Egypt
Checked for plagiarism: Yes
Review by: Single-blind
Copyright © 2022 Farshad Shahi, et al.
 This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Competing interests
The authors have declared that no competing interests exist.
Citation:
Introduction
One of the essential mechanism for testicular torsion and detorsion (TTD) have been known the testicular ischemia reperfusion injury (tIRI), which is a desperately serious urological emergency that can cause severe damage to testes, the main reproductive organ, and result in infertility 1, 2. Two important time periods have been expected for the occurrence of the disease, the first appearing before puberty, the second most commonly happens in the puberty. The timely and accurate diagnosis may prevent disease progression and deterioration 3. In Global statistics, it is estimated that its incidence would be around 1 in 4,000 young men population4. If the disease is identified in time, it will be possible to treat it to some extent with surgery and leading to blood reperfusion into testes. On the other hand, It is also possible that even the both testis often becomes completely dysfunctional with surgery 5. During Testicular torsion, the spermatic cord that conveys blood to the scrotum would be twisted. Eventually, the reduced blood flow initiates ischemia and following – detorsion --, the reperfusion generates reactive oxygen species (ROS)1, 3. Because of increase in generation of ROS and antioxidant defense to degrade excess ROS, the balance between ROS production and elimination is disturbed that all cellular components such as nucleic acids and proteins can be deeply affected by oxidative stress. As shown in many previous studies, it is obviously concluded to cause reduction sperm motility, plus DNA damage in the genome of spermatozoa and induces germ cell apoptosis (GCA)6, 7. It is well documented that ischemia reperfusion (IR) injury can strongly influence mitochondrial dysfunction , also the excessive production of free radicals can cause increase mitochondrial permeability transition pore (MPTP) opening, resulting in damage of membrane potential and extra free radical production, disorders in the mitochondrial respiratory chain and ATP production8, 9. The first study of Ozone therapy was reported by Wolff in 19741. Ozone therapy, a gas mixture of oxygen and ozone, has been referred to the process of managing ozone gas into your body to treat numerous of disease including wound infections, ischemic diseases, and joint problems 1, 2. As demonstrated in some studies, production of ATP has been stimulated by overproduction of oxidative carboxylation of pyruvate. Moreover, it can cause substantial -- decline in NADH level and oxidation of cytochrome C 3. Ozone acts as an alleviation of damage of oxidative stress -- by increasing GSH, SOD, CAT, and decreasing MDA, stimulating the antioxidant system of the cell and mitochondria 1, 2, 4.Our study suggested that the ozone-oxygen therapy exert the significant influence on testicular I/R injury in a rat after exposure to testicular torsion-detorsion.
Method and Materials
All chemicals and reagents were purchased from Sigma-Aldrich (Taufkrichen, Germany) in the best commercial grade.
Experimental Design
We used of animal and all protocols by Animal Experimentation Committee of Baquatollah University of Medical Sciences. The 40 male Sprague-Dawley rats -- weighing 250–300 g and 8 weeks old were purchased from Pasteur Institute (Tehran, Iran). Animals were homed in cages with a 12 hr light: 12 hr dark cycle at 25 ± 3 ∘C under normal environmental conditions and had free access to tap water and pellet diet. Animal’s anesthetization was performed by intra-peritoneal injection of 100 mg/kg ketamine hydrochloride combined with 20 mg/kg xylazine. Full experiments were done under aseptic environment. In the torsion/detorsion group, via a left-sided ilioinguinal cutting the left testicles were scooped. The left testis were whirled 720° in an anticlockwise direction and retained in this position through fixation by 11–0 silk suture to the scrotum and was reversed to the scrotum, and the cutting point was sealed. Testicular ischemia reperfusion injury is caused by testicular torsion-detorsion. In the treated group (control), the left testis was subjected with the same cutting mentioned above. The incised point was opened after 2 hours, and the testicles were rotated to the normal position. The testis were still viable and was inserted into the scrotum again 2. Orchiectomy operation in both testes was done on half of the animals after the recovery of torsion at 4 hours in each group. After one month, the animals were euthanized by cervical decapitation for collection of tissues. Animals were randomly assigned into 4 groups, including control, torsion/detortion, torsion/detortion + ozone/oxygen (30 µg/ml) and only ozone/oxygen, with the same surgical method-- done as in the torsion-detorsion group. Ozone-oxygen therapy (30 µg/ml) was syringed intravenously (IV) via the tail vein. The animal number in each group were 10.
Medozon
The Medozon serves for medical application (HAB company 2015, ozone generator, UMDNS-Nr.12899) was used for the generation of an oxygen/ozone mixture (Ozone/ Oxygen ratio: 99.95%/0.05%). The ozone / oxygen concentration can be adjusted from 5 to 80 µg/ml (ozone / oxygen concentration was regulated by spectrophotometry).
Sperm Isolation
At the end --, the animals were sacrificed and sperm were isolated from the left epididymis to analysis the kinematic parameters, vitality and characterization 10.
Sperm Analysis
Sample of Semen were collected by masturbation after 3–5 days of moderation. All samples were allowed to liquefy at 37˚C for 60 minutes and were then measured according to World Health Organization guidelines (WHO 2010). Following incubation for 1 hour at 37 ˚C in air, 10 ml of sperm suspension was placed on a Makler chamber and sperm motility parameters were analyzed by Computer Assisted Sperm Analysis (CASA). The semen analyzer used was the Hamilton Thorne Research semen analyzer (IVOS, Version 10.8x. Hamilton Thorne, Beverly, USA). The resulting variables were taken into reflection: ejaculate volume (mL), sperm concentration (106/mL), total sperm number (106/ejaculate), motility (%), and morphology (% abnormal forms). In addition to raw data on the percentage of motility, we also reflected absolute values in terms of motile sperm of millions per ejaculate (obtained by multiplying the total sperm per ejaculate by the percentage of sperm motility). Automated computer analysis of sperm motility (CASA System; Hamilton Thorne) was carried out on all Semen samples and included a heated stage at 37C. The variables taken into consideration were curvilinear velocity (VCL m/s), Beat frequency (BCF),Straightness= VSL/VAP×100)STR(,velocity of average nice if you have it path) VAP(, amplitude lateral head) ALH(,velocity of straight line)VSL) 11
Histological Evaluation
The tissues of testis were gathered for histological examinations. The sample were put in 10% formalin and inserted in paraffin then slice into 4 microns sections, and dyed with hematoxylin and eosin (H&E). The tissues were blindly evaluated under light microscope by a pathologist. Using Johnsen score, spermatogenesis and testicular injury were graded. All tissue sections are consistently analyzed and each is imputed a score from 1 to 10 12.
Testicular Tissue Preparation
All testicular tissue was separately located in a 1.5 mL centrifuge tube. Add 250 µL of 20 mM Tris-HCl (pH 7.5) ,1 mM EGTA, 150 mM NaCl, 1% NP–40, 1 mM Na2EDTA, 1 µg/ml leupeptin, 1 mM βglycerophosphate, 2.5 mM sodium pyrophosphate, 1 mM Na3VO4 and 1% sodium deoxycholate buffer with protease inhibitors. Lysates were then centrifuged at 11000 ×g at 4∘C for 10 min13.
Cell Viability
Cell viability was assessed by3-(4,5-dimethylthiazol-2-yl)-2,5- diphenyl tetrazolium bromide (MTT) staining as described by Mosmann. The MTT assay is based on the reduction of the tetrazolium salt, MTT, by viable cells. Spermatozoa (1×104 cells/well) were incubated in 96-wellplates in the presence or absence of Ozoe/Oxygen (O2/O3) for 48h in a final volume of 100 µl. At the end of the treatment, 20 µl of MTT (5mg/ml in PBS) was added to each well and incubated for an additional 4 h at 37 °C. The purple blue MTT formazan precipitate was dissolved in100 ml of DMSO and the absorbance was measured at 570nm using a ELISA reader (Tecan, Rainbow Thermo, Austria)14.
Quantification of Mitochondrial ROS Level
The ROS measurement was performed by flow cytometry using DCFH-DA. Briefly, isolated cells were incubated with, Ozone/ oxygen (30μg/ml) in ischemia and health groups in respiration buffer. a sample was taken and DCFH-DA was added (final concentration, 10 μm) to cells and was then incubated for 10 min. O2/O3 prevented ROS generation in isolated cells were determined through the flow cytometry (BD) equipped with a 488-nm argon ion laser and supplied with the softwaring 2.5. And the signals were obtained using a 530-nm band pass filter (FL-1 channel). Each determination is based on the mean fluorescence intensity of 10,000 counts15 .
GSSG and GSH Assay
GSSG and GSH were assayed pursuant to the spectrofluorometric method. Using different concentrations of GSSG and GSH, a standard calibration curve was drawn for each form of glutathioneand samples were assayed by a fluorimeter set at 350 nm excitation and 420 nm emission wavelengths 15.
Lipid Peroxidation Assay
Evaluation of MDA in testicular tissue were measured by measuring the level of MDA produced during the disintegration of lipid hydro peroxides by monitoring the absorbance at 532 nm by Beckman DU–7 spectrophotometer16 .
ADP/ATP Ratio Assay
The alterations of the ADP and ATP contents have been measured to differentiate modes of viability and cell death in testicular tissue by luminometer using of ADP and ATP Assay kit (MAK135 sigma, USA). ADP and ATP was done pursuant to the producer’s instructions16.
Cytochrome C Release Assay
Using the Quantikine Rat/Mouse Cytochrome C Immunoassay kit (Minneapolis, Minn) the 8 concentration of cytochrome C was measured. Cytochrome C amounts were done pursuant to the producer’s instructions.
Mitochondria Isolation
The testicular tissue was homogenate and spun down for at 2,000 g at 4 °C 10 min to throw away the pellet. Then supernatant was removed and suspended in HEPES buffer and 0.75 M sucrose and centrifuged at 10,000 g for 30 min. The supernatant was throwed away and the mitochondria pellets were collected in HEPES buffer and centrifuged at 10,000 g again for 10 min. then supernatant was also throwed away and the final pellet containing mitochondria was suspended againin phosphate buffered saline (PBS). It was stored at - 80 °C until use 14.
Dehydrogenase (Complex II) Activity
Mitochondrial succinate dehydrogenase (complex II) activity was measured by the reduction of MTT to formazan at 570 nm as described in previous studies 14.
Mitochondrial Membrane Potential Assay
Rhodamine 123 as a fluorescent cationic dye has been utilized for the measurement of mitochondrial membrane potential collapse. The mitochondria pellet (0.5 mg protein/mL) were suspended mitochondrial membrane potential assay buffer. With using of flow cytometry, the fluorescence intensity of rhodamine 123 was assayed 14.
Statistical Analysis
Findings are expressed as mean ± SED. Each experiment was done in triple, and the mean was utilized for statistical analysis. Statistical significance was defined using the one-way ANOVA tests, followed by the post hoc Tukey. Statistical significance was set at P < 0.05.
Results
Determination of Semen Collection
We isolated the sperm from the left epididymis and analyzed. We showed that semen characteristic parameters such as sperm number (×106/ejaculate), volume (ml), sperm concentration(M/ml) showed no changes in all groups but sperm viability (%) and normal sperm morphology (%) parameters significantly decreased in compared to control and torsion/detorsion+ozone/oxygen group. The results are presented in Table 1, Table 2.
Table 1. Analysis of semen collection| Parameter | C | C+30µg/ml | C.SCI | SCI.30 µg/ml |
| Sperm number (×10 6 /ejaculate) | 21±1 | 22±1 | 22±1.09 | 21±1 |
| Volume(ml) | 1.1±0.5 | 1.1±1 | 1.2±1 | 1.1±1 |
| Sperm viability (%) | 100±1.2 | 98±1 | 48±1.3*** | 100±1 |
| Sperm concentration(M/ml) | 40.11±1 | 41.22±1 | 38.66±1*** | 41.22±1.2 |
| Normal sperm morphology (%) | 100±1 | 97±1 | 78±1*** | 92±1 |
| Parameters | C | C+30 µg/ml | C.SCI | SCI.30 µg/ml |
| Fields | 4±1.1 | 4±1 | 4±1 | 4±1.5 |
| Quantity | 288±1.2 | 288±1.1 | 280±1 | 288±1 |
| Concentration (M/ml) | 15±1.3 | 15±1 | 15±1 | 15±1 |
| Class: A+B(PR)% | 77±1.4 | 67±1.2 | 32±1.1*** | 88±1 |
| Class: C(NP) % | 6±1 | 5±1 | 9±18*** | 3±1 |
| Class: D(IM) % | 14±1.7 | 11±1 | 65±1*** | 15±1 |
| Round Cell | 0 | 0 | 0 | 0 |
Determination of Sperm Motility and Kinetic Parameters
Sperm motility and kinetic parameter results are shown in Table 3. The results indicated remarkable changes in sperm motility and kinetic parameter in torsion/detorsion group compared to control and torsion/detorsion+ozone/oxygen group. There was a significant decrease in Linearity (LIN) = VSL/VCL × 100, Velocity of Straight Line (VSL), Velocity of Curved Line (VCL), Velocity of Average Path (VAP) and Straightness (STR) = VSL/VAP x 100. Compared to the control and torsion/detorsion+ozone/oxygen group, there was no significant statistical difference in Beat Frequency (BCF) and Lateral Amplitude (ALH) in torsion/detorsion group.
Table 3. Mean percentages kinematic parameters| Parameters | C | C+30 µg/ml | C.SCI | SCI.30 µg/ml |
| Class Name | A+B(PR) | A+B(PR) | Non motile | A+B(PR) |
| LIN, rel. units | 85.47±1 | 77.39±1 | 45.91±1*** | 87.71±1 |
| VSL, µm/s | 100±0.00 | 89±1.09 | 45±1.08*** | 100±1.2 |
| VCL, µm/s | 117±1.1 | 115±0.7 | 98±1.5** | 114±1.5 |
| VAP, µm/s | 107±1.5 | 115±1 | 41±1.11*** | 121±1 |
| ALH, µm | 1±1.2 | 1±1.1 | 1.8±1 | 1±1 |
| STR, rel. units | 0.99±1 | 0.87±1 | 0.55±1** | 0.84±1.3 |
| BCF, Hz | 4.11±1 | 4±1.5 | 4±1.2 | 4.1±1 |
Histopathological Evaluation
The results of the histopathological examination for each group are displayed in Figure 1, Figure 2. The presence of uniform seminiferous tubular morphology and there was normal testicular structure seen in the control and ozone/oxygen groups. In torsion/detorsion group, there was a severe distortion of tubules and a significant decrease in the diameter of the seminiferous tubular. Administration of ozone/oxygen protected the seminiferous tubular from damage after torsion/detorsion.
Figure 1.Torsion/detorsion surgical process: (A) before surgical, (B) after surgical.
Figure 2.Light microscope observations of H&E stained sections (×100). (A) positive control group: normal testicular architecture was seen. (B) Health group+ ozone/oxygen (30µg/ml). (C) torsion/detorsion. (D) torsion/detorsion + ozone/oxygen (30 µg/ml) therapy group.
Cell Viability
Figure 3 showed that injection ozone/oxygen with concentration of 5, 10, 20, 30, 40, 60 and 80 µg/ml in testicular cells. Otherwise of 5, 10, 20 and 40, 60 and 80 µg/ml concentration in torsion/detorsion group significantly decreased viability of cell compared to untreated control group but 30 µg/ml , we did not see any change level in viability testis cells in animal model.
Figure 3.Effect of Torsion /detorsion and ozone/oxygen on Cell viability in rat testis (A, B, C and D). Cytochrome c release in testis cells. As shown in graph all of concentration except 30 µg/ml induced decreased viability. ns indicates not significant difference with control group. ***indicates significant difference with control group.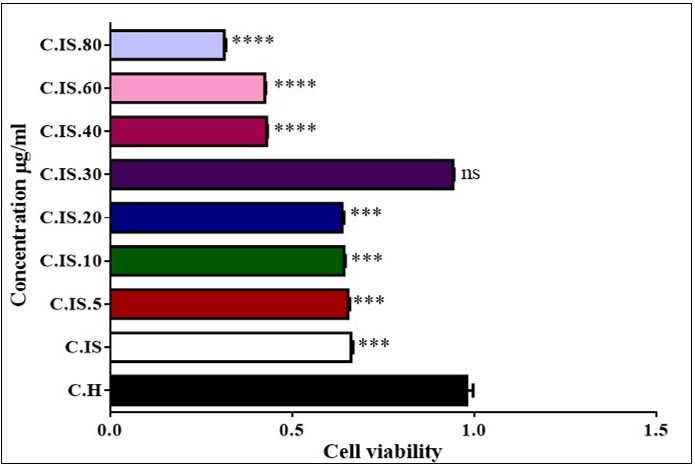
Cellular ROS Level
Figure 4, we showed that the rate of ROS formation in cells in torsion/detorsion group significantly raised compared to untreated control group.
Figure 4.Flowcytometric analysis of ROS formation in testis cell. Analysis were measured using BD flowcytometry. Fluoresce intensity mean of DA-DCF significantly increased in animal ischemic group in compared to control group that led to transporting pick from left to right of the histogram. Fluoresce intensity mean of DA-DCF. Significantly decreased in ischemic group + ozone/oxygen group treated group in compared to ischemic group group that led to transporting pick from right to left of the histogram.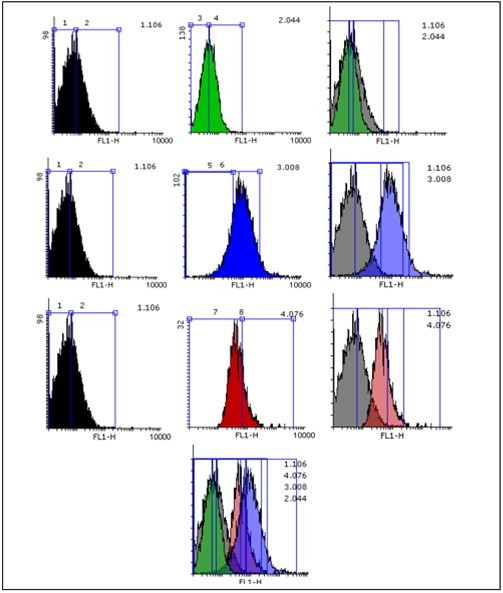
GSH and GSSG of Testicular Tissue
GHS level of testicular tissue has a significant decrease in 4 hours torsion/detorsion group comparing to control group (***p<0.001). Also, GSSG level of testicular tissue had a significant increase in torsion/detorsion group comparing to control group. These effects inhibited by oxygen/ozone therapy for both measured parameters (Figure 5 A and B).
Malondialdehyde Assay
Based on the results of Figure 5 C, MDA level of testicular tissue in torsion/detorsion group has significant increase comparing to control group (***p<0.001). MDA level of testicular tissue has a significant decrease ozone/oxygen-torsion/detorsion group comparing to torsion/detorsion group.
ADP/ATP Ratio Assay
As shown in Figure 5 D, ischemia reperfusion injury in torsion/detorsion group significantly decreased ATP content in testicular tissue. Animals treated with oxygen/ozone and torsion/detorsion showed a significant increase in the ATP content in comparison with the torsion/detorsion group.
Figure 5.Effect of Torsion /detorsion and ozone/oxygen on GSH, GSSG , MDA, ATP and cytochrome c release in rat testis (A, B, C ,D and E). MDA, GSH, GSSG ATP and cytochrome c release were measured in testicular tissues after treatment with Torsion /detorsion and ozone/oxygen. Graph A shows that content of GSH significantly (p<0.001) decreased in Torsion /detorsion group in compared to control group. The content of GSH not show any significant changes in Torsion /detorsion + ozone/oxygen and ozone/oxygen treated groups in compared to control group. Also, B graph indicates that content of GSSG significantly increased in Torsion /detorsion group in compared to control group. While there are not any significant changes in the content of GSSG in Torsion /detorsion + ozone/oxygen and ozone/oxygen treated groups in compared to control group. Similarly, Graphs C shows that lipid peroxidation significantly increased in Torsion /detorsion group in compared to control group while this effect inhibited in Torsion /detorsion + ozone / oxygen group. Graph D, ATP content decrease significantly in Torsion /detorsion group in compared to control group while this effect reflected in Torsion /detorsion + ozone/oxygen group. Ozone/oxygen alone did not show a significant effect. Graph E, Cytochrome c release in testis cells. As shown in graph Torsion /detorsion induced cytochrome c release and ozone/oxygen therapy significantly reflected this endpoint in cells. ns indicates not significant difference with control group. ***indicatessignificant difference with control group.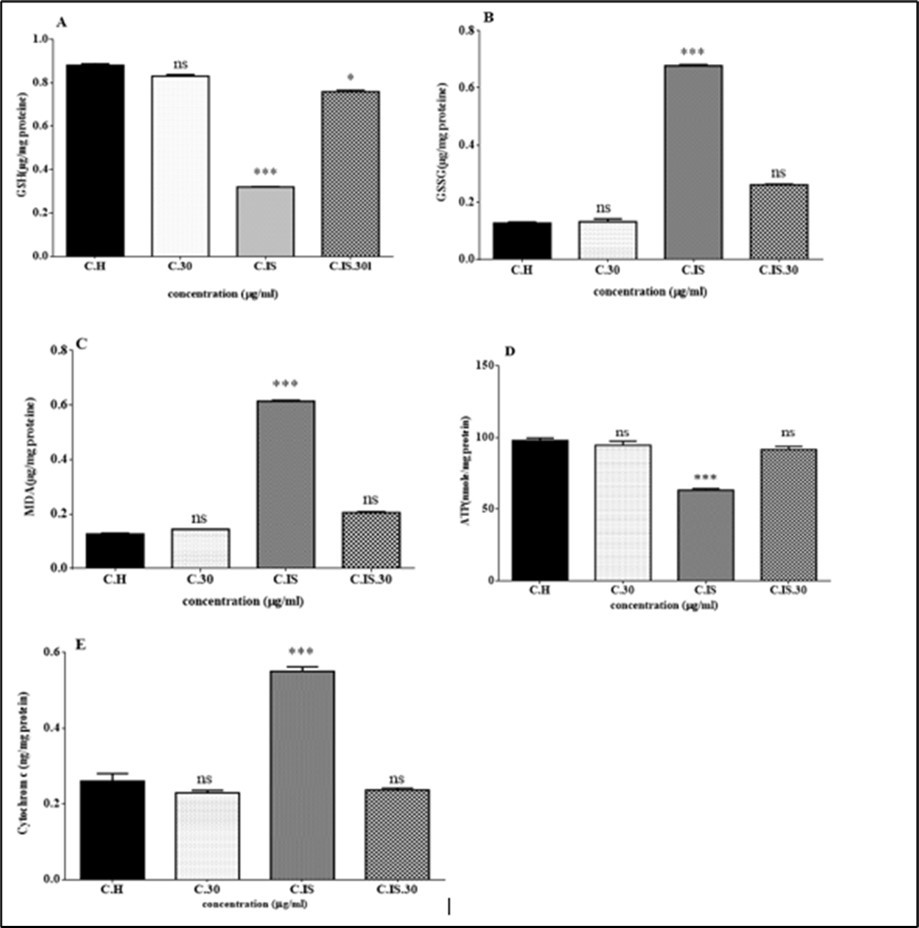
Cytochrome c Release
We examined cytochrome c release in the isolated cell obtained from testis in each group. Cytochrome C release was significantly raised in the isolated mitochondria in torsion/detorsion group in compared to control group. Moreover, cytochrome c release was inhibited with oxygen/ozone therapy (Figure 5E).
Succinate Dehydrogenase (Complex II) Activity
We examined Complex II in the isolated cell obtained from testis in each group. Complex II was significantly decreased in the isolated mitochondria in torsion/detorsion group in compared to control group. (Figure 6A)
Figure 6.Effect of Torsion /detorsion and ozone/oxygen on succinate activity and MMP in rat testis (A, B). these toxicity factors were measured in testicular tissues after treatment with Torsion /detorsion and ozone/oxygen. Graphs A shows that succinate activity significantly decreased in ischemic group in compared to control group while this effect inhibited in TD + ozone / oxygen group. Graph B shows that MMP significantly (p<0.001) increased in ischemic group in compared to control group.
Mitochondrial Membrane Potential Collapse
The redistribution of the rhodamine 123 into the cytosol has been utilized for assessment of the MMP collapse as the known indicator of mitochondrial damage which subsequent leads to mitochondrial membrane permeability transition (MPT). As shown in Figure 6B, there was a remarkable increase in the rhodamine 123 redistributions in torsion/detorsion group compared to control group.
Discussion
This study was designed to evaluate the protective effects of ozone-oxygen therapy on testicular ischemiareperfusion injury (IRI) in a rat after induction of testicular torsion (TT). Research has shown that oxidative stress plays a main role in the pathologic mechanism underlying IRI of the testis 1, 3. Testicles due to high levels of unsaturated fatty acids and also ROS are sensitive to oxidative stress process 3. Oxidative stress is a condition in which the level of ROS increases compared to the level of cellular antioxidants 4, 6. Research has shown that reperfusion injury (RI) occurs in two phases. The first phase is associated with mitochondrial dysfunction and an excessive increase in the generation of ROS7. High levels of ROS are associated with irreversible damage to macromolecules including proteins, lipids and DNA 8, 17. IRI involves the generation of ROS, apoptosis and lipid peroxidation (LPO). Antioxidants are known as one of the most important lines of protection of the organism against side effect of IRI in the testicular cell environment. Also, various antioxidants have been used to prevent damage caused by oxidative stress in IRI 3. Ozone therapy is one of the most important methods used to prevent IRI. Furthermore, the positive effects of ozone therapy on IRI have been studied in different tissues and organs 1, 18, 19. Studies have shown that ozone therapy is associated with a decrease in the level of ROS and subsequent oxidative stress, a decrease in the level of LPO and ATP depletion and an increase in the level of activity of cellular antioxidants, including glutathione (GSH) 1, 2, 7, 19, 20. The results showed that ozone therapy was able to reduce the level of ROS in testicular mitochondria in the testicular torsion group. The results of our study are in agreement with previous studies that have shown that ozone therapy has the ability to reduce the ROS generation and oxidative stress 8, 17, 18. ROS as one of the mitochondrial products can affect the mitochondria by collapsing in the MMP. Collapse in the MMP is considered to be one of the events during which caspase cascade activation occurs, resulting in irreversible cell death 21, 22. Ozone therapy has been able to reduce the collapse of the MMP in the mitochondria of testicular tissue in the testicular torsion group. The results suggest that ozone therapy reduces mitochondrial dysfunction by reducing the level of ROS and subsequently improving the MMP collapse. In biological systems, the evaluation of GSH changes is considered as one of the important indicators of oxidative stress. Our results showed that ozone therapy causes an increase in GSH levels and other antioxidant enzymes and a decrease in GSSG levels in the testicular torsion group 7, 9. These results are in agreement with the results of another study that showed that ozone therapy is associated with an increase in the level of intracellular antioxidant enzymes 1, 9. LPO is considered as one of the oxidation products and indicators of cellular toxicity 9, 18. Reduction in MDA levels in the testicular torsion group treated with ozone compared to the testicular torsion group indicates the therapeutic effects of ozone in reduction of LPO. Research has shown that there is a direct relationship between the level of ROS and LPO 23, 24. The results show that ozone therapy has reduced LPO levels by decreasing the level of ROS and increasing the level of cellular antioxidants in testicular tissue in the testicular torsion. Mitochondrial dysfunction is known as a consequence of oxidative stress during IRI. In addition, oxidative damage in mitochondria is associated with changes in its structure and function, including mitochondrial swelling and cytochrome c release and eventually cell death 1, 3. Furthermore, ozone therapy has been able to reduce mitochondrial damage and cell death in the heart IRI by increasing the level of the antioxidant system as well as inhibiting the release of cytochrome c, caspase cascade and apoptotic signaling 1, 25. In this study, a reduction in mitochondrial damage, mitochondrial swelling and cytochrome c release was observed in the in the testicular torsion group treated with ozone. These results are in agreement with previous studies 1. During the process of apoptosis, the release of cytochrome c is considered as one of the most important events 26, 27.
Conclusion
In this study, we injected ozone/oxygen to the testicular ischemiareperfusion (IRI) rat animal model. However , our results showed that a higher concentration of ozone/oxygen caused electron transfer chain impairment, which leads to decreased cell viability , but we injected 30 μg to animal model and ozone/oxygen decreased ROS production, lipid peroxidation ,GSSG and ADP and cytochrome c release compared with IRI animal group. Our study suggests that mitochondrial dysfunction and the uncoupling of oxidative phosphorylation may play key roles in the etiology of testicular ischemia. In general, the present study results confirmed that a moderate concentration of ozone (30 μg) can be considered as a protective in animal rat model.
References
- 1.H A Cai. (2020) Ozone alleviates ischemia/reperfusion injury by inhibiting mitochondrion‐mediated apoptosis pathway in SH‐SY5Y cells. Cell biology international. 44(4), 975-984.
- 2.Mete F. (2017) Comparison of intraperitoneal and intratesticular ozone therapy for the treatment of testicular ischemia-reperfusion injury in rats. Asian journal of andrology. 19(1), 43.
- 3.Vaos G, Zavras N. (2017) Antioxidants in experimental ischemia-reperfusion injury of the testis: Where are we heading towards? World journal of methodology. 7(2), 37.
- 4.Elsurer C.Postconditioning Ozone Alleviates Ischemia-Reperfusion Injury and Enhances Flap Endurance in Rats. , Journal of Investigative Surgery 33(1), 15-24.
- 5.Hameister R. (2020) Reactive oxygen/nitrogen species (ROS/RNS) and oxidative stress in arthroplasty. , Journal of Biomedical Materials Research Part B: Applied Biomaterials 108(5), 2073-2087.
- 6.J Y Lee. (2020) Erigeron annuus protects PC12 neuronal cells from oxidative stress induced by ROS-mediated apoptosis. Evidence-Based Complementary and Alternative Medicine.
- 7.Ekici S. (2012) Comparison of melatonin and ozone in the prevention of reperfusion injury following unilateral testicular torsion in rats. Urology. 80(4), 899-906.
- 8.Aydogdu I. (2019) Does ozone administration have a protective or therapeutic effect against radiotherapy-induced testicular injury? Journal of cancer research and therapeutics. 15(8), 76.
- 9.H A EROĞLU. (2020) . Effects of Ozone and L-Carnitine on Kidney MDA, GSH, and GSHPx Levels in Acetaminophen Toxicity. KAFKAS ÜNİVERSİTESİ VETERİNER FAKÜLTESİ DERGİSİ 26-1.
- 10.Adamkovicova M. (2016) Sperm motility and morphology changes in rats exposed to cadmium and diazinon. Reproductive Biology and Endocrinology. 14(1), 42.
- 11.Oh S.Effect of adding taurine, hypotaurine and trehalose as antioxidants to a tris-based egg yolk extender on korean jeju black bull sperm quality following cryopreservation. , Journal of Animal Science and Technology 54(4), 283-290.
- 12.Zhou X-L. (2014) Protective effects of lipoxin A4 in testis injury following testicular torsion and detorsion in rats. Mediators of inflammation.
- 13.C D Pederzolli. (2007) 5-Oxoproline reduces non-enzymatic antioxidant defenses in vitro in rat brain. Metabolic brain disease. 22(1), 51-65.
- 14.Zhang F. (2008) In vitro effect of manganese chloride exposure on energy metabolism and oxidative damage of mitochondria isolated from rat brain. Environmental toxicology and pharmacology. 26(2), 232-236.
- 15.Seydi E. (2015) Involvement of mitochondrial-mediated caspase-3 activation and lysosomal labilization in acrylamide-induced liver toxicity. Toxicological & Environmental Chemistry. 97(5), 563-575.
- 16.Salimi A. (2016) Toxicity of methyl tertiary-butyl ether on human blood lymphocytes. Environmental Science and Pollution Research 23(9), 8556-8564.
- 17.Kal A. (2017) The protective effect of prophylactic ozone administration against retinal ischemia-reperfusion injury. Cutaneous and ocular toxicology. 36(1), 39-47.
- 18.Jiang B. (2020) Protective effects of ozone oxidative postconditioning on long-term injury after renal ischemia/reperfusion in rat. in Transplantation Proceedings.
- 19.Haj B. (2014) Effect of ozone on intestinal recovery following intestinal ischemia–reperfusion injury in a rat. Pediatric surgery international. 30(2), 181-188.
- 20.A E El-Mehi, M A Faried. (2020) Controlled ozone therapy modulates the neurodegenerative changes in the frontal cortex of the aged albino rat. Annals of Anatomy-Anatomischer Anzeiger. 227-151428.
- 21.Yang S. (2020) Sesamin induces A549 cell mitophagy and mitochondrial apoptosis via a reactive oxygen species-mediated reduction in mitochondrial membrane potential. The Korean journal of physiology & pharmacology: official journal of the Korean Physiological Society and the Korean Society of Pharmacology. 24(3), 223.
- 22.Salehcheh M. (2020) Multi-walled carbon nanotubes induce oxidative stress, apoptosis, and dysfunction in isolated rat heart mitochondria: protective effect of naringin. Environmental Science and Pollution Research 1-10.
- 23.Gao H.CYP4A11 is involved in the development of nonalcoholic fatty liver disease via ROSinduced lipid peroxidation and inflammation. , International Journal of Molecular Medicine 45(4), 1121-1129.
- 24.A S Menhali. (2020) Lipid peroxidation is involved in calcium dependent upregulation of mitochondrial metabolism in skeletal muscle. Biochimica et Biophysica Acta (BBA)-General Subjects. 1864(3), 129487.
- 25.Scassellati C. (2020) Molecular mechanisms in cognitive frailty: potential therapeutic targets for oxygen-ozone treatment. Mechanisms of Ageing and Development. 186, 111210.
